Abstract
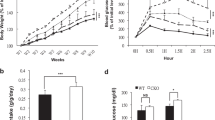
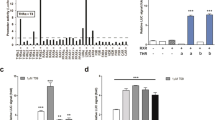
In obesity and type 2 diabetes, expression of the GLUT4 glucose transporter is decreased selectively in adipocytes. Adipose-specific Glut4 (also known as Slc2a4) knockout (adipose-Glut4-/-) mice show insulin resistance secondarily in muscle and liver. Here we show, using DNA arrays, that expression of retinol binding protein-4 (RBP4) is elevated in adipose tissue of adipose-Glut4-/- mice. We show that serum RBP4 levels are elevated in insulin-resistant mice and humans with obesity and type 2 diabetes. RBP4 levels are normalized by rosiglitazone, an insulin-sensitizing drug. Transgenic overexpression of human RBP4 or injection of recombinant RBP4 in normal mice causes insulin resistance. Conversely, genetic deletion of Rbp4 enhances insulin sensitivity. Fenretinide, a synthetic retinoid that increases urinary excretion of RBP4, normalizes serum RBP4 levels and improves insulin resistance and glucose intolerance in mice with obesity induced by a high-fat diet. Increasing serum RBP4 induces hepatic expression of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase (PEPCK) and impairs insulin signalling in muscle. Thus, RBP4 is an adipocyte-derived ‘signal’ that may contribute to the pathogenesis of type 2 diabetes. Lowering RBP4 could be a new strategy for treating type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Despres, J. P. et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 334, 952–957 (1996)
Shepherd, P. R. & Kahn, B. B. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N. Engl. J. Med. 341, 248–257 (1999)
DeFronzo, R. A. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 5, 171–269 (1997)
Shepherd, P. R. et al. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J. Biol. Chem. 268, 22243–22246 (1993)
Abel, E. D. et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 (2001)
Tozzo, E., Gnudi, L. & Kahn, B. B. Amelioration of insulin resistance in streptozotocin diabetic mice by transgenic overexpression of GLUT4 driven by an adipose-specific promoter. Endocrinology 138, 1604–1611 (1997)
Carvalho, E., Kotani, K., Peroni, O. & Kahn, B. B. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am. J. Physiol. Endocrinol. Metab. doi:10.1152/ajpendo.0016.2005 (2005)
Kershaw, E. E. & Flier, J. S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556 (2004)
Quadro, L. et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 18, 4633–4644 (1999)
Minokoshi, Y., Kahn, C. R. & Kahn, B. B. Tissue-specific ablation of the GLUT4 glucose transporter or the insulin receptor challenges assumptions about insulin action and glucose homeostasis. J. Biol. Chem. 278, 33609–33612 (2003)
Kitamura, T., Kahn, C. R. & Accili, D. Insulin receptor knockout mice. Annu. Rev. Physiol. 65, 313–332 (2003)
Blaner, W. S. Retinol-binding protein: the serum transport protein for vitamin A. Endocr. Rev. 10, 308–316 (1989)
Kotani, K., Peroni, O. D., Minokoshi, Y., Boss, O. & Kahn, B. B. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J. Clin. Invest. 114, 1666–1675 (2004)
Masuzaki, H. et al. A transgenic model of visceral obesity and the metabolic syndrome. Science 294, 2166–2170 (2001)
Minokoshi, Y. et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569–574 (2004)
Nikoulina, S. E. et al. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes 49, 263–271 (2000)
Lee, C. H., Olson, P. & Evans, R. M. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144, 2201–2207 (2003)
Kotani, K., Kim, Y. B., Peroni, O., Mundt, A. & Kahn, B. B. Rosiglitazone treatment normalizes glucose tolerance in adipose-specific GLUT4 knockout mice but renders muscle-specific GLUT4 knockout more diabetic [abstract]. Diabetes 50, A274 (2001)
Quadro, L. et al. Muscle expression of human retinol-binding protein (RBP). Suppression of the visual defect of RBP knockout mice. J. Biol. Chem. 277, 30191–30197 (2002)
Naylor, H. M. & Newcomer, M. E. The structure of human retinol-binding protein (RBP) with its carrier protein transthyretin reveals an interaction with the carboxy terminus of RBP. Biochemistry 38, 2647–2653 (1999)
Kato, M., Kato, K., Blaner, W. S., Chertow, B. S. & Goodman, D. S. Plasma and cellular retinoid-binding proteins and transthyretin (prealbumin) are all localized in the islets of Langerhans in the rat. Proc. Natl Acad. Sci. USA 82, 2488–2492 (1985)
Malpeli, G., Folli, C. & Berni, R. Retinoid binding to retinol-binding protein and the interference with the interaction with transthyretin. Biochim. Biophys. Acta 1294, 48–54 (1996)
Mothe, I. & Van Obberghen, E. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J. Biol. Chem. 271, 11222–11227 (1996)
Anai, M. et al. Enhanced insulin-stimulated activation of phosphatidylinositol 3-kinase in the liver of high-fat-fed rats. Diabetes 48, 158–169 (1999)
Pagliassotti, M. J., Kang, J., Thresher, J. S., Sung, C. K. & Bizeau, M. E. Elevated basal PI 3-kinase activity and reduced insulin signalling in sucrose-induced hepatic insulin resistance. Am. J. Physiol. Endocrinol. Metab. 282, E170–E176 (2002)
Shin, D. J., Odom, D. P., Scribner, K. B., Ghoshal, S. & McGrane, M. M. Retinoid regulation of the phosphoenolpyruvate carboxykinase gene in liver. Mol. Cell. Endocrinol. 195, 39–54 (2002)
Kahn, C. R., Lauris, V., Koch, S., Crettaz, M. & Granner, D. K. Acute and chronic regulation of phosphoenolpyruvate carboxykinase mRNA by insulin and glucose. Mol. Endocrinol. 3, 840–845 (1989)
Wang, J. C., Stafford, J. M., Scott, D. K., Sutherland, C. & Granner, D. K. The molecular physiology of hepatic nuclear factor 3 in the regulation of gluconeogenesis. J. Biol. Chem. 275, 14717–14721 (2000)
Basualdo, C. G., Wein, E. E. & Basu, T. K. Vitamin A (retinol) status of First Nation adults with non-insulin-dependent diabetes mellitus. J. Am. Coll. Nutr. 16, 39–45 (1997)
Abahusain, M. A., Wright, J., Dickerson, J. W. & de Vol, E. B. Retinol, alpha-tocopherol and carotenoids in diabetes. Eur. J. Clin. Nutr. 53, 630–635 (1999)
Meigs, J. B., Panhuysen, C. I., Myers, R. H., Wilson, P. W. & Cupples, L. A. A genome-wide scan for loci linked to plasma levels of glucose and HbA1c in a community-based sample of Caucasian pedigrees: The Framingham Offspring Study. Diabetes 51, 833–840 (2002)
Duggirala, R. et al. Linkage of type 2 diabetes mellitus and of age at onset to a genetic location on chromosome 10q in Mexican Americans. Am. J. Hum. Genet. 64, 1127–1140 (1999)
Tsutsumi, C. et al. Retinoids and retinoid-binding protein expression in rat adipocytes. J. Biol. Chem. 267, 1805–1810 (1992)
Zovich, D. C. et al. Differentiation-dependent expression of retinoid-binding proteins in BFC-1β adipocytes. J. Biol. Chem. 267, 13884–13889 (1992)
Pedersen, O., Kahn, C. R. & Kahn, B. B. Divergent regulation of the Glut 1 and Glut 4 glucose transporters in isolated adipocytes from Zucker rats. J. Clin. Invest. 89, 1964–1973 (1992)
Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 10, 940–954 (1996)
Koistinen, H. A. et al. Dyslipidemia and a reversible decrease in insulin sensitivity induced by therapy with 13-cis-retinoic acid. Diabetes Metab. Res. Rev. 17, 391–395 (2001)
Rodondi, N. et al. High risk for hyperlipidemia and the metabolic syndrome after an episode of hypertriglyceridemia during 13-cis retinoic acid therapy for acne: a pharmacogenetic study. Ann. Intern. Med. 136, 582–589 (2002)
Kliewer, S. A., Xu, H. E., Lambert, M. H. & Willson, T. M. Peroxisome proliferator-activated receptors: from genes to physiology. Recent Prog. Horm. Res. 56, 239–263 (2001)
Sivaprasadarao, A. & Findlay, J. B. The interaction of retinol-binding protein with its plasma-membrane receptor. Biochem. J. 255, 561–569 (1988)
Matarese, V. & Lodish, H. F. Specific uptake of retinol-binding protein by variant F9 cell lines. J. Biol. Chem. 268, 18859–18865 (1993)
Christensen, E. I. et al. Evidence for an essential role of megalin in transepithelial transport of retinol. J. Am. Soc. Nephrol. 10, 685–695 (1999)
Berni, R., Clerici, M., Malpeli, G., Cleris, L. & Formelli, F. Retinoids: in vitro interaction with retinol-binding protein and influence on plasma retinol. FASEB J. 7, 1179–1184 (1993)
Monaco, H. L. The transthyretin-retinol-binding protein complex. Biochim. Biophys. Acta 1482, 65–72 (2000)
Sheikh, M. S. et al. N-(4-hydroxyphenyl)retinamide (4-HPR)-mediated biological actions involve retinoid receptor-independent pathways in human breast carcinoma. Carcinogenesis 16, 2477–2486 (1995)
Um, S. J. et al. Antiproliferative mechanism of retinoid derivatives in ovarian cancer cells. Cancer Lett. 174, 127–134 (2001)
Shen, Q., Cline, G. W., Shulman, G. I., Leibowitz, M. D. & Davies, P. J. Effects of rexinoids on glucose transport and insulin-mediated signalling in skeletal muscles of diabetic (db/db) mice. J. Biol. Chem. 279, 19721–19731 (2004)
Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council. Nutrient Requirements of Laboratory Animals 4th revised edn, 92 (National Academies Press, Washington DC, 1995)
Wang, T. T., Lewis, K. C. & Phang, J. M. Production of human plasma retinol-binding protein in Escherichia coli. Gene 133, 291–294 (1993)
Xie, Y., Lashuel, H. A., Miroy, G. J., Dikler, S. & Kelly, J. W. Recombinant human retinol-binding protein refolding, native disulfide formation, and characterization. Protein Expr. Purif. 14, 31–37 (1998)
Acknowledgements
We are indebted to W. S. Blaner and M. Gottesman for RBP4-overexpressing and Rbp4 knockout mice; T. Ciaraldi and R. R. Henry for human serum samples and metabolic data; Takeda Pharmaceutical Company Limited for recombinant mouse RBP4 and M. Fujisawa for sharing unpublished data; the BIDMC DNA Array Facility for assisting with the Affimetrix array; V. Petkova for assistance with Taqman; E. Rosen, Y.-B. Kim and Y. Minokoshi for helpful suggestions; and C. Wason for technical help. We thank J. E. Smith and B. Mickelson for advice regarding incorporation of fenretinide into rodent diets. Fenretinide was provided by the R. W. Johnson Pharmaceutical Company with the assistance of the National Cancer Institute's Cancer Therapy Evaluation Program. This work was supported by grants from the NIH (B.B.K and W. Blaner), Takeda Pharmaceutical Company Limited (B.B.K.) and the American Diabetes Association (B.B.K.). T.E.G. and J.M.Z. were supported by NIH career awards, F.P. by a Swiss National Science Foundation fellowship, and N.M. by an American Heart Association fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The project was partially supported by a research grant from Takeda Pharmaceutical Company Limited (Osaka, Japan).
Supplementary information
Supplementary Methods
Methods, including animal husbandry and diet; microarray analysis; measurement of metabolic parameters, insulin tolerance tests (ITT), glucose tolerance tests (GTT); and expression and purification of recombinant hRBP4. (DOC 27 kb)
Supplementary Tables S1-S2
Supplementary Table S1 shows the clinical parameters of human subjects. Supplementary Table S2 shows metabolic parameters of RBP4 transgenic and Rbp4 knockout mice. (DOC 72 kb)
Supplementary Figures S1-S2
Supplementary Figure S1 shows a correlation between serum RBP4 levels and insulin levels in obese mice on a high-fat diet. Supplementary Figure S2 shows western blot data comparing serum RBP4 levels in the mouse models that were studied. (PPT 34 kb)
Rights and permissions
About this article
Cite this article
Yang, Q., Graham, T., Mody, N. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 (2005). https://doi.org/10.1038/nature03711
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03711
This article is cited by
-
Assessing the Impact of Bariatric Surgery on Retinol-Binding Protein 4 (RBP4): A Systematic Review and Meta-Analysis
Obesity Surgery (2024)
-
Review of lipocalin-2-mediated effects in diabetic retinopathy
International Ophthalmology (2024)
-
Preoperative sarcopenia combined with prognostic nutritional index predicts long-term prognosis of radical gastrectomy with advanced gastric cancer: a comprehensive analysis of two-center study
BMC Cancer (2023)
-
The relationship between NAFLD and retinol-binding protein 4 - an updated systematic review and meta-analysis
Lipids in Health and Disease (2023)
-
Shift work is associated with an increased risk of type 2 diabetes and elevated RBP4 level: cross sectional analysis from the OHSPIW cohort study
BMC Public Health (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.