Abstract
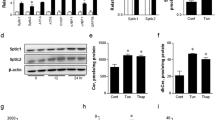
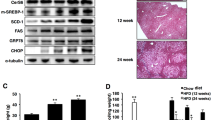
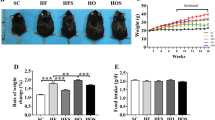
The endoplasmic reticulum (ER) is the main site of protein and lipid synthesis, membrane biogenesis, xenobiotic detoxification and cellular calcium storage, and perturbation of ER homeostasis leads to stress and the activation of the unfolded protein response1. Chronic activation of ER stress has been shown to have an important role in the development of insulin resistance and diabetes in obesity2. However, the mechanisms that lead to chronic ER stress in a metabolic context in general, and in obesity in particular, are not understood. Here we comparatively examined the proteomic and lipidomic landscape of hepatic ER purified from lean and obese mice to explore the mechanisms of chronic ER stress in obesity. We found suppression of protein but stimulation of lipid synthesis in the obese ER without significant alterations in chaperone content. Alterations in ER fatty acid and lipid composition result in the inhibition of sarco/endoplasmic reticulum calcium ATPase (SERCA) activity and ER stress. Correcting the obesity-induced alteration of ER phospholipid composition or hepatic Serca overexpression in vivo both reduced chronic ER stress and improved glucose homeostasis. Hence, we established that abnormal lipid and calcium metabolism are important contributors to hepatic ER stress in obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev. Mol. Cell Biol. 8, 519–529 (2007)
Hotamisligil, G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010)
Oyadomari, S. et al. Dephosphorylation of translation initiation factor 2α enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 7, 520–532 (2008)
Erbay, E. et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nature Med. 15, 1383–1391 (2009)
Li, Y. et al. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 279, 37030–37039 (2004)
Borradaile, N. M. et al. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 47, 2726–2737 (2006)
Kim, S. J. et al. Omega-3 and omega-6 fatty acids suppress ER- and oxidative stress in cultured neurons and neuronal progenitor cells from mice lacking PPT1. Neurosci. Lett. 479, 292–296 (2010)
Cheng, K. H., Lepock, J. R., Hui, S. W. & Yeagle, P. L. The role of cholesterol in the activity of reconstituted Ca-ATPase vesicles containing unsaturated phosphatidylethanolamine. J. Biol. Chem. 261, 5081–5087 (1986)
Miyauchi, Y. et al. Comprehensive analysis of expression and function of 51 sarco(endo)plasmic reticulum Ca2+-ATPase mutants associated with Darier disease. J. Biol. Chem. 281, 22882–22895 (2006)
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006)
Kammoun, H. L. et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 119, 1201–1215 (2009)
Jacobs, R. L. et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J. Biol. Chem. 285, 22403–22413 (2010)
Lodish, H. F. & Kong, N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J. Biol. Chem. 265, 10893–10899 (1990)
Gregor, M. F. et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700 (2009)
Kars, M. et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59, 1899–1905 (2010)
Park, S. W., Zhou, Y., Lee, J. & Ozcan, U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc. Natl Acad. Sci. USA 107, 19320–19325 (2010)
Li, S. Y. et al. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia 49, 1434–1446 (2006)
Cardozo, A. K. et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 54, 452–461 (2005)
Li, G. et al. Role of ERO1-α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J. Cell Biol. 186, 783–792 (2009)
Schiller, J. et al. Lipid analysis of human HDL and LDL by MALDI-TOF mass spectrometry and (31)P-NMR. J. Lipid Res. 42, 1501–1508 (2001)
Brown, M. S. & Goldstein, J. L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008)
Cox, B. & Emili, A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nature Protocols 1, 1872–1878 (2006)
Moore, L., Chen, T., Knapp, H. R., Jr & Landon, E. J. Energy-dependent calcium sequestration activity in rat liver microsomes. J. Biol. Chem. 250, 4562–4568 (1975)
Cao, H. et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933–944 (2008)
Acknowledgements
We thank A. Porter, E. Freeman and R. Davis for technical assistance. The anti-HERP antibody is a gift of Y. Hirabayashi. We thank the members of the G.S.H. laboratory for scientific discussions and critical reading of the manuscript. This work was supported in part by the National Institutes of Health (DK52539 and 1RC4-DK090942) and a research grant from Syndexa Pharmaceuticals to G.S.H. S.F. was supported in part by the NIH/NIEHS postdoctoral training grant (T32ES007155).
Author information
Authors and Affiliations
Contributions
S.F. designed, performed experiments, analysed and interpreted the results and wrote the manuscript; L.Y. and P.L. performed some animal experiments; O.H., L.D., W.H. and X.L. performed statistical and bioinformatic analysis of the proteomic data; S.W.M quantified the lipid composition of ER and analysed the data; A.R.I. analysed the protein composition of ER; G.S.H. generated the hypothesis, designed the project, analysed and interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.S.H. is a shareholder of Syndexa Pharmaceuticals and serves on the Scientific Advisory Board. A patent application has been filed using technologies discussed in this paper.
Supplementary information
Supplementary Information
The file contains Supplementary Methods, Supplementary References, Supplementary Figures 1-7 with legends and Supplementary Tables 2-6. (PDF 1187 kb)
Supplementary Table 1
The file contains Supplementary Table 1 (XLS 1103 kb)
Rights and permissions
About this article
Cite this article
Fu, S., Yang, L., Li, P. et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473, 528–531 (2011). https://doi.org/10.1038/nature09968
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09968
This article is cited by
-
TOLLIP inhibits lipid accumulation and the integrated stress response in alveolar macrophages to control Mycobacterium tuberculosis infection
Nature Microbiology (2024)
-
Phosphoinositides and intracellular calcium signaling: novel insights into phosphoinositides and calcium coupling as negative regulators of cellular signaling
Experimental & Molecular Medicine (2023)
-
Candesartan, an angiotensin-II receptor blocker, ameliorates insulin resistance and hepatosteatosis by reducing intracellular calcium overload and lipid accumulation
Experimental & Molecular Medicine (2023)
-
Control of immune cell function by the unfolded protein response
Nature Reviews Immunology (2023)
-
Lysosomal Ca2+ as a mediator of palmitate-induced lipotoxicity
Cell Death Discovery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.