Abstract
Haematopoietic stem and progenitor cells are maintained by special microenvironments known as niches in bone marrow1,2,3,4,5,6. Many studies have identified diverse candidate cells that constitute niches for haematopoietic stem cells in the marrow, including osteoblasts7,8,9,10, endothelial cells11,12,13,14, Schwann cells15, α-smooth muscle actin-expressing macrophages16 and mesenchymal progenitors such as CXC chemokine ligand (CXCL)12-abundant reticular (CAR) cells17,18, stem cell factor-expressing cells13, nestin-expressing cells19 and platelet-derived growth factor receptor-α (PDGFR-α)+Sca-1+CD45−Ter119− (PαS) cells20. However, the molecular basis of the formation of the niches remains unclear. Here we find that the transcription factor Foxc1 is preferentially expressed in the adipo-osteogenic progenitor CAR cells essential for haematopoietic stem and progenitor cell maintenance in vivo5,13,18 in the developing and adult bone marrow. When Foxc1 was deleted in all marrow mesenchymal cells or CAR cells, from embryogenesis onwards, osteoblasts appeared normal, but haematopoietic stem and progenitor cells were markedly reduced and marrow cavities were occupied by adipocytes (yellow adipose marrow) with reduced CAR cells. Inducible deletion of Foxc1 in adult mice depleted haematopoietic stem and progenitor cells and reduced CXCL12 and stem cell factor expression in CAR cells but did not induce a change to yellow marrow. These data suggest a role for Foxc1 in inhibiting adipogenic processes in CAR progenitors. Foxc1 might also promote CAR cell development, upregulating CXCL12 and stem cell factor expression. This study identifies Foxc1 as a specific transcriptional regulator essential for development and maintenance of the mesenchymal niches for haematopoietic stem and progenitor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008)
Li, L. & Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545 (2010)
Ehninger, A. & Trumpp, A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med. 208, 421–428 (2011)
Bianco, P. Bone and the hematopoietic niche: a tale of two stem cells. Blood 117, 5281–5288 (2011)
Nagasawa, T., Omatsu, Y. & Sugiyama, T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends Immunol. 32, 315–320 (2011)
Mercier, F. E., Ragu, C. & Scadden, D. T. The bone marrow at the crossroads of blood and immunity. Nature Rev. Immunol. 12, 49–60 (2011)
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003)
Calvi, L. M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003)
Arai, F. et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161 (2004)
Sugimura, R. et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 150, 351–365 (2012)
Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C. & Morrison, S. J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005)
Butler, J. M. et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264 (2010)
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012)
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013)
Yamazaki, S. et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158 (2011)
Ludin, A. et al. Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nature Immunol. 13, 1072–1082 (2012)
Sugiyama, T., Kohara, H., Noda, M. & Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 (2006)
Omatsu, Y. et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399 (2010)
Mendez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010)
Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013)
Nagasawa, T. et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638 (1996)
Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007)
Chan, C. K. et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494 (2009)
Morikawa, S. et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 206, 2483–2496 (2009)
Kume, T. et al. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell 93, 985–996 (1998)
Sasman, A. et al. Generation of conditional alleles for Foxc1 and Foxc2 in mice. Genesis 50, 766–774 (2012)
Logan, M. et al. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77–80 (2002)
Naveiras, O. et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263 (2009)
Kodama, H., Nose, M., Niida, S. & Nishikawa, S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp. Hematol. 22, 979–984 (1994)
Nehls, M. et al. Two genetically separable steps in the differentiation of thymic epithelium. Science 272, 886–889 (1996)
Ara, T. et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19, 257–267 (2003)
Rodda, S. J. & McMahon, A. P. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133, 3231–3244 (2006)
DeFalco, J. et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 291, 2608–2613 (2001)
Ruzankina, Y. et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 (2007)
Koni, P. A. et al. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med. 193, 741–754 (2001)
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neurosci. 13, 133–140 (2010)
Joe, A. W. et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature Cell Biol. 12, 153–163 (2010)
Uezumi, A., Fukada, S., Yamamoto, N., Takeda, S. & Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature Cell Biol. 12, 143–152 (2010)
Harrison, D. E., Jordan, C. T., Zhong, R. K. & Astle, C. M. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp. Hematol. 21, 206–219 (1993)
Morita, S., Kojima, T. & Kitamura, T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7, 1063–1066 (2000)
Acknowledgements
We appreciate the technical assistance provided by K. Kawaguchi and G. Kondoh, and thank I. Sasagawa for secretarial assistance. This research was supported by JST, CREST and the Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI.
Author information
Authors and Affiliations
Contributions
Y.O. and T.N. designed and performed the experiments, analysed the data and prepared the paper. T.N. supervised the study. M.S. and T.S. performed the experiments. T.K. contributed materials and tools. All authors discussed results and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Progenitors of CAR cells and their development in fetal and postnatal bone marrow.
a, b, Immunohistochemical analysis of E14.5 (a) and E16.5 (b) femurs of Osx–GFP mice with antibodies against PDGFR-β (red) and CD31 (blue). Osx–GFP+ cells (green) expressing PDGFR-β (arrowheads) were observed inside the marrow cavity at E16.5 (b with magnified views of the boxed area on the right) but not at E14.5 (a). B, cortical bone (b, right). c, Immunohistochemical analysis of the newborn femur of CXCL12–GFP mice with antibodies against PDGFR-β (red). CXCL12–GFP+ cells (green) express PDGFR-β. d, Immunofluorescent profiles of Osx–GFP+PDGFR-βhi cells (boxed area) in the limbs of E14.5 and E16.5 Osx–GFP mice. e–g Relative mRNA expression levels of PDGFR-β, Lepr, CXCL12, SCF, PPARγ, C/EBPα and Osx in Osx–GFP+PDGFR-βhi, Osx–GFP+PDGFR-βlo, CXCL12–GFP+ cells, osteoblasts (Ob), PαS, Osx–GFP−PDGFR-β+Sca-1−CD31−CD45−Ter119− (Osx−Rβ+) cells and endothelial cells (ECs) from femurs of E16.5 Osx–GFP mice and newborn CXCL12–GFP mice (e) as well as 14- to 20-week-old CXCL12–GFP mice (g), or in CXCL12–GFP+ cells from newborn, 1-week-old, 3-week-old and 14- to 20-week-old CXCL12–GFP mice (f) (n = 3). *P < 0.05.
Extended Data Figure 2 Expression of PDGFR-α in Sca-1+CD31− cells.
Immunohistochemical analysis of the bone marrow cavity of wild-type mice with antibodies against Sca-1 (red), CD31 (blue) and PDGFR-α (green). All Sca-1+CD31− mesenchymal cells surrounding arteries expressed PDGFR-α.
Extended Data Figure 3 Expression levels of Foxc2 were similar in CAR cells and osteoblasts.
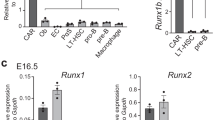
a–c, Relative mRNA expression levels of Foxc2 in CAR cells, osteoblasts (Ob), PαS cells, bone marrow endothelial cells (ECs), c-kit+Sca-1+Lin− (KSL) cells, macrophages and muscle PαS cells from adult mice (a), in Osx–GFP+PDGFR-βhi, Osx–GFP+PDGFR-βlo, CXCL12–GFP+ CAR cells, osteoblasts, PαS, Osx–GFP−PDGFR-β+Sca-1−CD31−CD45−Ter119− (Osx−Rβ+) cells and endothelial cells from femurs of E16.5 Osx–GFP mice (b) and newborn mice (b, c), and in CAR cells and osteoblasts from newborn, 1-week-old, 3-week-old and 14- to 20-week-old CXCL12–GFP mice (c) (n = 3). Error bars, s.d. *P < 0.05.
Extended Data Figure 4 The numbers of functional multilineage reconstituting HSCs were markedly reduced in the bone marrow of Prx1-Cre;Foxc1f/f mice.
a, C-kit+Sca-1+Lin− (KSL) cells sorted from 3-week-old control or Prx1-Cre;Foxc1f/f mice as tester progenitors and those sorted from wild-type mice as competitor progenitors were mixed at a ratio of 1:1 and injected intravenously into recipient mice. The percentages of donor-derived Gr-1+ myeloid, B220+ B and CD3+ T cells in peripheral blood were analysed for 14 weeks after transplantation (n = 3). b, The numbers of KSL cells in the bone marrow of 3-week-old control or Prx1-Cre;Foxc1f/f mice (n = 3). Because KSL cell numbers were reduced, a marked decrease in the numbers of functional multilineage reconstituting HSCs in the mutants was observed. c, The numbers of LTC-ICs in the bone marrow of 3-week-old control and Prx1-Cre;Foxc1f/f mice (n = 3). LTC-IC numbers per femurs and tibiae were assayed by limiting dilution analysis. Error bars, s.d. *P < 0.05.
Extended Data Figure 5 The numbers of HSPCs were reduced in newborn Prx1-Cre;Foxc1f/f mice although to a lesser extent than juvenile mutants.
Total haematopoietic cell counts and the numbers of cells in the CD150+CD48− KSL population (HSCs), pro-B cells, pre-B cells and proerythroblasts (pro-E) in the bone marrow of newborn control and Prx1-Cre;Foxc1f/f mice (n = 5). *P < 0.05.
Extended Data Figure 6 CAR cells can be identified as S100+ cells in the marrow cavity.
Immunohistochemical analysis of bone marrow from CXCL12–GFP mice with antibodies against S100 (red). CAR cells (green) were identified as S100+ cells in the marrow cavity.
Extended Data Figure 7 Expression of PDGFR-α in Sca-1+CD31− PαS cells in the bone marrow from Prx1-Cre;Foxc1f/f or Lepr-Cre;Foxc1f/f mice.
a, b, Immunohistochemical analysis of the bone marrow cavity of control, Prx1-Cre;Foxc1f/f (a) and Lepr-Cre;Foxc1f/f mice (b) with antibodies against Sca-1 (red), CD31 (blue) and PDGFR-α (green). Sca-1+CD31− mesenchymal cells surrounding arteries expressed PDGFR-α (arrowheads).
Extended Data Figure 8 The numbers of functional multilineage reconstituting HSCs were markedly reduced in the bone marrow of Lepr-Cre;Foxc1f/f mice.
a, KSL cells sorted from 14- to 18-week-old control or Lepr-Cre;Foxc1f/f mice as tester progenitors and those sorted from wild-type mice as competitor progenitors were mixed at a ratio of 1:1 and injected intravenously into recipient mice. The percentages of donor-derived Gr-1+ myeloid, B220+ B and CD3+ T cells in peripheral blood were analysed for 14 weeks after transplantation (n = 3). b, The numbers of KSL cells in the bone marrow of 14- to 18-week-old control or Lepr-Cre;Foxc1f/f mice (n = 3). Because KSL cell numbers were reduced, a marked decrease in the numbers of functional multilineage reconstituting HSCs in the mutants was observed. c, The numbers of LTC-ICs in the bone marrow of 14- to 18-week-old control or Lepr-Cre;Foxc1f/f mice (n = 3). LTC-IC numbers per femur and tibia were assayed by limiting dilution analysis. Error bars, s.d. *P < 0.05.
Extended Data Figure 9 HSPC maintenance and haematopoiesis were not affected when Foxc1 was deleted from endothelial cells and haematopoietic cells.
Total haematopoietic cell counts and the numbers of LT-HSCs, ST-HSCs, MPPs, CLPs, pro-B cells, pre-B cells, proerythroblasts (pro-E) and granulocyte/macrophage progenitors (GMPs) in the bone marrow of 14- to 18-week-old control and Tie2-Cre;Foxc1f/f mice, in which the Foxc1 gene was deleted in endothelial cells and haematopoietic cells (n = 3).
Rights and permissions
About this article
Cite this article
Omatsu, Y., Seike, M., Sugiyama, T. et al. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature 508, 536–540 (2014). https://doi.org/10.1038/nature13071
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13071
This article is cited by
-
Dietary restriction plus exercise change gene expression of Cxcl12 abundant reticular cells in female mice
Journal of Bone and Mineral Metabolism (2024)
-
Cellular niches for hematopoietic stem cells in bone marrow under normal and malignant conditions
Inflammation and Regeneration (2023)
-
Novel maternal duplication of 6p22.3-p25.3 with subtelomeric 6p25.3 deletion: new clinical findings and genotype–phenotype correlations
Molecular Cytogenetics (2023)
-
Spatial heterogeneity of bone marrow endothelial cells unveils a distinct subtype in the epiphysis
Nature Cell Biology (2023)
-
Ebf3+ niche-derived CXCL12 is required for the localization and maintenance of hematopoietic stem cells
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.