Abstract
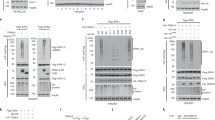
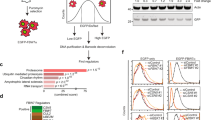
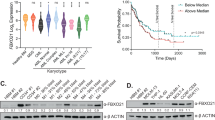
The covalent attachment of ubiquitin to target proteins influences various cellular processes, including DNA repair, NF-κB signalling and cell survival1. The most common mode of regulation by ubiquitin-conjugation involves specialized ubiquitin-binding proteins that bind to ubiquitylated proteins and link them to downstream biochemical processes. Unravelling how the ubiquitin-message is recognized is essential because aberrant ubiquitin-mediated signalling contributes to tumour formation2. Recent evidence indicates that inhibitor of apoptosis (IAP) proteins are frequently overexpressed in cancer and their expression level is implicated in contributing to tumorigenesis, chemoresistance, disease progression and poor patient-survival3. Here, we have identified an evolutionarily conserved ubiquitin-associated (UBA) domain in IAPs, which enables them to bind to Lys 63-linked polyubiquitin. We found that the UBA domain is essential for the oncogenic potential of cIAP1, to maintain endothelial cell survival and to protect cells from TNF-α-induced apoptosis. Moreover, the UBA domain is required for XIAP and cIAP2–MALT1 to activate NF-κB. Our data suggest that the UBA domain of cIAP2–MALT1 stimulates NF-κB signalling by binding to polyubiquitylated NEMO. Significantly, 98% of all cIAP2–MALT1 fusion proteins retain the UBA domain, suggesting that ubiquitin-binding contributes to the oncogenic potential of cIAP2–MALT1 in MALT lymphoma. Our data identify IAPs as ubiquitin-binding proteins that contribute to ubiquitin-mediated cell survival, NF-κB signalling and oncogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Di Fiore, P. P., Polo, S. & Hofmann, K. When ubiquitin meets ubiquitin receptors: a signalling connection. Nature Rev. Mol. Cell Biol. 4, 491–497 (2003).
Hoeller, D., Hecker, C. M. & Dikic, I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nature Rev. Cancer 6, 776–788 (2006).
Hunter, A. M., LaCasse, E. C. & Korneluk, R. G. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 12, 1543–1568 (2007).
Cook, W. J., Jeffrey, L. C., Sullivan, M. L. & Vierstra, R. D. Three-dimensional structure of a ubiquitin-conjugating enzyme (E2). J. Biol. Chem. 267, 15116–15121 (1992).
Varadan, R. et al. Solution conformation of Lys 63-linked di-ubiquitin chain provides clues to functional diversity of polyubiquitin signaling. J. Biol. Chem. 279, 7055–7063 (2004).
Salvesen, G. S. & Duckett, C. S. Apoptosis: IAP proteins: blocking the road to death's door. Nature Rev. Mol. Cell Biol. 3, 401–410. (2002).
Vince, J. E. et al. IAP antagonists target cIAP1 to induce TNF-α-dependent apoptosis. Cell 131, 682–693 (2007).
Varfolomeev, E. et al. IAP antagonists induce auto-ubiquitination of c-IAPs, NF-κB activation, and TNF-α-dependent apoptosis. Cell 131, 669–681 (2007).
Petersen, S. L. et al. Autocrine TNF-α signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 12, 445–456 (2007).
Li, X., Yang, Y. & Ashwell, J. D. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature 416, 345 (2002).
Isaacson, P. G. & Du, M. Q. Gastrointestinal lymphoma: where morphology meets molecular biology. J. Pathol. 205, 255–274 (2005).
Hofer-Warbinek, R. et al. Activation of NF-κ B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J. Biol. Chem. 275, 22064–22068 (2000).
Gaither, A. et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 67, 11493–11498 (2007).
Bertrand, M. J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008).
Lucas, P. C. et al. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-κB signaling pathway. J. Biol. Chem. 276, 19012–19019 (2001).
Kirkin, V. & Dikic, I. Role of ubiquitin- and Ubl-binding proteins in cell signaling. Curr. Opin. Cell Biol. 19, 199–205 (2007).
Swanson, K. A., Hicke, L. & Radhakrishnan, I. Structural basis for mono-ubiquitin recognition by the Ede1 UBA domain. J. Mol. Biol. 358, 713–724 (2006).
Ohno, A. et al. Structure of the UBA domain of Dsk2p in complex with ubiquitin molecular determinants for ubiquitin recognition. Structure 13, 521–532 (2005).
Raasi, S., Orlov, I., Fleming, K. G. & Pickart, C. M. Binding of polyubiquitin chains to ubiquitin-associated (UBA) domains of HHR23A. J. Mol. Biol. 341, 1367–1379 (2004).
Raasi, S., Varadan, R., Fushman, D. & Pickart, C. M. Diverse polyubiquitin interaction properties of ubiquitin-associated domains. Nature Struct. Mol. Biol. 12, 708–714 (2005).
Silke, J. et al. The anti-apoptotic activity of XIAP is retained upon mutation of both the caspase-3- and caspase-9-interacting sites. J. Cell Biol. 157, 115–124 (2002).
Santoro, M. M., Samuel, T., Mitchell, T., Reed, J. C. & Stainier, D. Y. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nature Genet. 39, 1397–1402 (2007).
Zender, L. et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125, 1253–1267 (2006).
Zender, L. et al. Generation and analysis of genetically defined liver carcinomas derived from bipotential liver progenitors. Cold Spring Harb. Symp. Quant. Biol. 70, 251–261 (2005).
Eckelman, B. P., Salvesen, G. S. & Scott, F. L. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 7, 988–994 (2006).
Birkey Reffey, S., Wurthner, J. U., Parks, W. T., Roberts, A. B. & Duckett, C. S. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-β signaling. J. Biol. Chem. 276, 26542–26549 (2001).
Yamaguchi, K. et al. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 18, 179–187 (1999).
Lu, M. et al. XIAP induces NF-κB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol. Cell 26, 689–702 (2007).
Peschard, P. et al. Structural basis for ubiquitin-mediated dimerization and activation of the ubiquitin protein ligase Cbl-b. Mol. Cell 27, 474–485 (2007).
Zhou, H., Du, M. Q. & Dixit, V. M. Constitutive NF-κB activation by the t(11;18)(q21;q21) product in MALT lymphoma is linked to deregulated ubiquitin ligase activity. Cancer Cell 7, 425–431 (2005).
Noels, H. et al. A Novel TRAF6 binding site in MALT1 defines distinct mechanisms of NF-κB activation by API2middle dotMALT1 fusions. J. Biol. Chem. 282, 10180–10189 (2007).
Zhou, H. et al. Bcl10 activates the NF-κB pathway through ubiquitination of NEMO. Nature 427, 167–171 (2004).
Sun, L., Deng, L., Ea, C. K., Xia, Z. P. & Chen, Z. J. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 (2004).
Baens, M., Steyls, A., Dierlamm, J., De Wolf-Peeters, C. & Marynen, P. Structure of the MLT gene and molecular characterization of the genomic breakpoint junctions in the t(11;18)(q21;q21) of marginal zone B-cell lymphomas of MALT type. Genes Chromosomes Cancer 29, 281–291 (2000).
Young, S. S. et al. Genomic organization and physical map of the human inhibitors of apoptosis: HIAP1 and HIAP2. Mamm. Genome 10, 44–48 (1999).
Lucas, P. C. et al. A dual role for the API2 moiety in API2-MALT1-dependent NF-κB activation: heterotypic oligomerization and TRAF2 recruitment. Oncogene 26, 5643–5654 (2007).
Wu, C. J., Conze, D. B., Li, T., Srinivasula, S. M. & Ashwell, J. D. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-κB activation [corrected]. Nature Cell Biol. 8, 398–406 (2006).
Silke, J. et al. Determination of cell survival by RING-mediated regulation of inhibitor of apoptosis (IAP) protein abundance. Proc. Natl Acad. Sci. USA 102, 16182–16187 (2005).
Hu, S. et al. cIAP2 is a ubiquitin protein ligase for BCL10 and is dysregulated in mucosa-associated lymphoid tissue lymphomas. J. Clin. Invest. 116, 174–181 (2006).
Li, M. et al. An essential role of the NF-κ B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 166, 7128–7135 (2001).
Acknowledgements
We would like to thank Xiaolu Yang, David Komander, David Barford, Thomas Farkas, Ivan Dikic and Marja Jaattela for reagents, discussions and invaluable technical support. We thank Irene Scarfò for help with the cIAP1 zebrafish experiments, and Hyejin Cho and Beicong Ma for excellent technical assistance and help with the mouse tumour model. We thank Alan Ashworth and members of the Meier laboratory for critical reading of the manuscript and helpful discussions. We thank Vishva Dixit for sharing unpublished results. M.G.-H. is supported by a fellowship from the Danish Cancer Society. M.M.S. is supported by a HFSP Career Developmental Award, Fondazione San Paolo and Regione Piemonte.
Author information
Authors and Affiliations
Contributions
M.G.-H. and M.D. performed all experiments, except for those in Fig. 4; M.M. and J.S. planned and performed the cIAP1−/− MEF reconstitution assay; M.M.S. planned and performed the zebrafish reconstitution assay; L.Z., W.X. and S.L. designed, performed and supervised the cIAP1 mouse tumour assay; T.T. provided various constructs and technical support; P.C.A.F. performed sequence alignments and database searches with structural prediction algorithms; J.M.B and M.Z. performed 3D modelling and sequence analysis; M.G.-H. and P.M. designed and supervised the study and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 5765 kb)
Rights and permissions
About this article
Cite this article
Gyrd-Hansen, M., Darding, M., Miasari, M. et al. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-κB as well as cell survival and oncogenesis. Nat Cell Biol 10, 1309–1317 (2008). https://doi.org/10.1038/ncb1789
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1789
This article is cited by
-
TRAF2/3 deficient B cells resist DNA damage-induced apoptosis via NF-κB2/XIAP/cIAP2 axis and IAP antagonist sensitizes mutant lymphomas to chemotherapeutic drugs
Cell Death & Disease (2023)
-
Drice restrains Diap2-mediated inflammatory signalling and intestinal inflammation
Cell Death & Differentiation (2022)
-
Increased migration and motility in XIAP-null cells mediated by the C-RAF protein kinase
Scientific Reports (2022)
-
XIAP as a multifaceted molecule in Cellular Signaling
Apoptosis (2022)
-
Met1-linked ubiquitin signalling in health and disease: inflammation, immunity, cancer, and beyond
Cell Death & Differentiation (2021)