Abstract
CD4+ T-helper cells producing interleukin-17 (IL-17), known as T-helper 17 (TH17) cells, comprise heterogeneous subsets that exhibit distinct pathogenicity. Although pathogenic and non-pathogenic TH17 subsets share a common RORγt-dependent TH17 transcriptional programme, transcriptional regulatory mechanisms specific to each of these subsets are mostly unknown. Here we show that the AP-1 transcription factor JunB is critical for TH17 pathogenicity. JunB, which is induced by IL-6, is essential for expression of RORγt and IL-23 receptor by facilitating DNA binding of BATF at the Rorc locus in IL-23-dependent pathogenic TH17 cells, but not in TGF-β1-dependent non-pathogenic TH17 cells. Junb-deficient T cells fail to induce TH17-mediated autoimmune encephalomyelitis and colitis. However, JunB deficiency does not affect the abundance of gut-resident non-pathogenic TH17 cells. The selective requirement of JunB for IL-23-dependent TH17 pathogenicity suggests that the JunB-dependent pathway may be a therapeutic target for autoimmune diseases.
Similar content being viewed by others
Introduction
Interleukin-17 (IL-17)-producing T-helper 17 (TH17) cells, exhibiting heterogeneous pathogenicity, serve diverse biological functions1,2,3. Pathogenic TH17 cells have a central function in autoimmune and chronic inflammatory diseases4,5,6, whereas non-pathogenic TH17 cells, which accumulate in the gut at steady state, are probably involved in gut homoeostasis and host defence7,8,9. Pathogenicity of TH17 cells is associated with cytokine signals. A subset of TH17 cells induced in the presence of transforming growth factor β1 (TGF-β1) and IL-6 (hereafter referred to as TH17(β))10,11,12 is non-pathogenic, because transfer of the cells into mice induces weak or no experimental autoimmune encephalomyelitis (EAE)13,14. On the other hand, another subset of TH17 cells generated in the presence of IL-6, IL-1β and IL-23 (hereafter referred to as TH17(23)) is highly pathogenic, and transfer of these cells into mice induces severe EAE13,14. Furthermore, it is broadly accepted that IL-23 is needed for TH17 cell pathogenicity and that it can induce pathogenicity in previously non-pathogenic TH17 cells15,16,17,18. Indeed, mice deficient in IL-23 signalling are resistant to EAE and chronic colitis15,17,19,20, but they have normal numbers of gut-resident TH17 cells at steady state21. The critical function of IL-23 signalling for TH17 pathogenicity is also evidenced by a correlation between IL-23 mutation and human autoimmune diseases22.
Pathogenic and non-pathogenic TH17 cells differentially express a number of genes. For example, anti-inflammatory IL-10 is specifically induced in TH17(β) cells, whereas inflammatory granulocyte–macrophage colony-stimulating factor (GM-CSF) is preferentially produced by TH17(23) cells13,14,23,24,25 to contribute to the pathogenicity of TH17 cells26,27. However, pathogenic and non-pathogenic TH17 cells express a subset of molecules (TH17 signature genes) comparably, including Il17a, Il17f and Il23 receptor (Il23r)13,23,25. Induction of these TH17 signature genes is regulated by transcription factors including basic leucine zipper ATF-like transcription factor (BATF), interferon regulatory factor 4 (IRF4), signal transducer and activator of transcription 3 (STAT3), and RORγt in both pathogenic and non-pathogenic TH17 cells28,29,30,31,32. BATF and IRF4 are induced by T-cell receptor signalling and bind to loci of a large number of genes, including TH17 signature genes, where they promote chromatin accessibility29,33,34. Under TH17-polarizing conditions, cytokine signals activate STAT3 and induce RORγt, both of which bind to loci of TH17 genes occupied by BATF and IRF4 and activate TH17 gene transcription28,29. IL-6 activates STAT3 through JAK-mediated phosphorylation35,36. However, it is unclear how RORγt induction is regulated by different combinations of cytokines under TH17(β)- and TH17(23)-polarizing conditions.
Here we show that, in an RNAi screen of transcription factors involved in IL-23 signalling, an AP-1 transcription factor, JunB, is required for IL-23-dependent gene induction. Additional analyses show that JunB is required for induction of RORγt in pathogenic TH17(23) cells, but not in non-pathogenic TH17(β) cells. Mechanistically, JunB facilitates DNA binding of BATF, IRF4 and STAT3 at multiple gene loci including Rorc (encoding RORγt) and Il17a under TH17(23)-polarizing conditions. Furthermore, we show that JunB is essential for pathogenicity of TH17 cells in EAE and colitis models, but it is not required for generation of non-pathogenic, gut-resident TH17 cells. These data suggest that the JunB-dependent pathway is required for IL-23-dependent pathogenicity of TH17 cells.
Results
Identification of JunB as a regulator of IL-23 signalling
TGF-β1 signalling is associated with non-pathogenic TH17 differentiation, whereas IL-23 signalling facilitates pathogenicity of TH17 cells13,15,16,17,19. However, transcriptional mechanisms underlying control of TH17 pathogenicity in the presence of these cytokines remain to be fully determined. To better understand IL-23-dependent transcriptional regulation, we attempted to identify transcription factors responsible for expression of genes promoted by IL-23 signalling in TH17 cells. Based on published microarray data13,23, we selected 263 transcription factors that are highly expressed in TH17 cells (Supplementary Data 1). Retroviruses expressing shRNAs against these transcription factors were individually transduced into TH17 cells generated under pathogenic TH17(23)-polarizing conditions (in the presence of IL-6, IL-1β and IL-23). To evaluate the effect of transcription factor knockdown on IL-23-dependent signalling, we measured levels of ectonucleotide pyrophosphate/phosphodiesterase 2 (Enpp2) mRNA because we found that induction of Enpp2 was significantly facilitated by IL-23 stimulation (Supplementary Fig. 1a). Enpp2 induction was most heavily diminished by knockdown of an AP-1 transcription factor, JunB (Supplementary Fig. 1b). RNAi ablation of JunB also significantly reduced expression of a TH17 signature molecule, Il23r (Supplementary Fig. 1c). JunB interacts with another AP-1 family member, BATF, an essential transcription factor for TH17 differentiation31,33,34, suggesting that JunB might be involved in TH17 differentiation; however, the physiological functions of JunB in TH17 differentiation remain unknown.
JunB is induced in TH17 cells
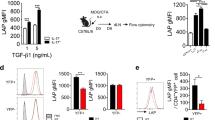
We first examined JunB expression in TH17 cells differentiated in vitro. Immunoblot analysis of JunB showed that naive CD4+ T cells activated under TH17(β)-polarizing conditions (in the presence of TGF-β1 and IL-6) or TH17(23)-polarizing conditions expressed higher levels of JunB compared to T cells activated under neutral conditions (in the absence of cytokines) (TH0) or induced T regulatory (iTreg)-polarizing conditions (in the presence of TGF-β1 and IL-2) (Fig. 1a). There was no detectable JunB expression in naive CD4+ T cells (Supplementary Fig. 1d). Quantitative reverse transcription–polymerase chain reaction (qRT–PCR) analysis also showed an increase in Junb mRNA levels in both TH17(β) and TH17(23) cells (Fig. 1b). We also found that IL-6 stimulation was sufficient to augment JunB expression, whereas neither IL-1β nor IL-23 signalling significantly affected JunB expression in activated CD4+ T cells (Fig. 1c,d). IL-6 signalling is mediated by STAT3 (refs 35, 36). Indeed, JunB induction was severely impaired in Stat3-deficient cells under TH17(β)-polarizing conditions (Fig. 1e), indicating that STAT3 is required for JunB induction in TH17 cells. These results suggest that JunB expression is facilitated in TH17 cells in an IL-6- and STAT3-dependent manner.
(a,b) Naive CD4+ T cells were activated with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β1 and IL-6 (TH17(β)), IL-6, IL-1β and IL-23 (TH17(23)), TGF-β1 and IL-2 (iTreg) or in the absence of cytokines (TH0) for 60 h. JunB protein (a) and mRNA (b) were detected by immunoblot and qRT–PCR, respectively. mRNA results were normalized to Hprt mRNA. Error bars indicate s.d. (n=4). Asterisks indicate significant differences (P<0.05) by unpaired two-tailed Student’s t-test. (c,d) Immunoblot analysis of JunB in CD4+ T cells activated in the presence of the indicated cytokines for 60 h. (e) Naive CD4+ T cells from Stat3fl/fl mice were activated, infected with Cre-expressing retrovirus, then cultured under TH17(β)-polarizing conditions for 60 h. JunB was detected by immunoblot analysis. (a,c,e) Data represent two independent experiments. (b,d) Data represent three independent experiments.
JunB regulates TH17 differentiation in vitro
To determine the importance of JunB in TH17 differentiation, we generated T-cell-specific Junb-deficient (Cd4CreJunbfl/fl) mice (Supplementary Fig. 2). Loss of JunB did not affect the abundance of CD4+ and CD8+ T cells, nor did it affect that of naive (CD62LhiCD44lo), effector (CD62LhiCD44hi) and memory (CD62LloCD44hi) CD4+ T-cell populations in lymph nodes (LNs) and spleens (Supplementary Fig. 3).
To evaluate the function of JunB in TH17 differentiation in vitro, we activated Junb-deficient naive CD4+ T cells under TH17-polarizing conditions. Notably, IL-17A expression was severely diminished in Junb-deficient CD4+ T cells under pathogenic TH17(23)-polarizing conditions (Fig. 2a). However, a substantial number of Junb-deficient CD4+ T cells produced IL-17A, albeit less than that produced by control cells, under non-pathogenic TH17(β)-polarizing conditions (Fig. 2a). Loss of JunB promoted production of a TH1 signature cytokine, IFN-γ, under TH17(23) conditions (Fig. 2a). Moreover, expression of a TH17 pathogenic cytokine, GM-CSF, was also augmented in the absence of JunB (Supplementary Fig. 4a). Consistent with its impact on production of IL-17A, induction of an essential TH17 transcription factor, RORγt, was severely impaired in Junb-deficient T cells under TH17(23)-polarizing conditions, but less strikingly under TH17(β)-polarizing conditions (Fig. 2b and Supplementary Fig. 4b). Normal mRNA expression of IL-6 receptor was observed in Junb-deficient cells under TH17(β) conditions, while it was slightly elevated under TH17(23) conditions (Supplementary Fig. 4c), suggesting that the defective TH17(23) differentiation in Junb-deficient cells may not be due to impaired IL-6 receptor expression.
(a,b) Naive CD4+ T cells from Cd4CreJunbfl/fl and control Junbfl/fl mice were activated under TH17(β)- or TH17(23)-polarizing conditions for 3 days, and expression of IL-17A and IFN-γ was analysed by flow cytometry (a). RORγt was detected by immunoblot analysis (b). (c) Naive CD4+ T cells from Cd4CreJunbfl/fl and control Junbfl/fl mice were activated in the presence of TGF-β3 and IL-6 (TH17(β3)) for 3 days, and expression of IL-17A and IFN-γ was analysed by flow cytometry. (d) Cd4CreJunbfl/fl and control cells were activated under TH17(β)-polarizing conditions for 3 days, and IL-17-high and IL-17-low populations were sorted using the IL-17-capture method. Enrichment of IL-17-high cells was confirmed by detecting IL-17A expression on re-stimulation immediately after sorting. Cells were then cultured in the presence of IL-23 alone for another 2 days. Production of IL-17A and IFN-γ was assessed by flow cytometry. (a,c) Data represent three independent experiments. (b,d) Data represent two independent experiments.
Remarkably, expression of the Treg master transcription factor, forkhead box protein 3 (Foxp3)37, significantly increased in Junb-deficient CD4+ T cells under TH17(β) conditions, but not under TH17(23) or iTreg conditions (Supplementary Fig. 4b,d), suggesting that JunB is involved in IL-6-mediated suppression of TGF-β-dependent Foxp3 induction. In addition, JunB deficiency also resulted in induction of Foxp3 and a slight reduction of IL-17A production under other TH17-polarizing conditions (the presence of IL-6 and TGF-β3; TH17(β3)) (Fig. 2c and Supplementary Fig. 4e). Foxp3 suppresses IL-17A production by antagonizing functions of RORγt38,39. Indeed, the abundance of IL-17A-expressing cells in a population with low Foxp3 expression (Foxp3-low population) was greater than in a population with high Foxp3 expression (Foxp3-high population) in Junb-deficient cells activated under TH17(β) conditions (Supplementary Fig. 4f), suggesting that defective IL-17A production in Junb-deficient TH17(β) cells might be partly due to aberrant induction of Foxp3.
Since IL-23 promotes pathogenicity of TH17(β) cells14, we investigated the function of JunB in IL-23-stimulated TH17(β) cells. When we activated CD4+ T cells in the presence of TGF-β1 and IL-6, with or without IL-23, IL-23 did not affect IL-17A and IFN-γ production in either Junb-deficient or control cells (Supplementary Fig. 4g). We next examined the impact of JunB deficiency on IL-23-stimulated TH17(β) cells in the absence of TGF-β1. We activated Junb-deficient T cells under TH17(β) conditions for 3 days and sorted them into IL-17-high or IL-17-low populations using an IL-17-capture method for further culturing with IL-23 alone. Although JunB deficiency did not affect expression of IL-17A on re-stimulation immediately after sorting, further culturing of IL-17-high cells with IL-23 significantly decreased the abundance of IL-17A-expressing cells in Junb-deficient cells, but not in controls (Fig. 2d). The data also showed a marked increase in the proportion of IFN-γ single-producing cells in the absence of JunB (Fig. 2d). Interestingly, IL-23 facilitated IL-17A expression in the IL-17-low populations of control cells, but not in Junb-deficient cells (Fig. 2d). Thus, JunB seems to have an important function in IL-23-dependent maintenance of TH17 cells in the absence of TGF-β1.
JunB-dependent transcriptional regulation in TH17 cells
To further clarify functions of JunB in TH17 differentiation, we performed a microarray analysis of Junb-deficient and control CD4+ T cells activated under TH17(β)- or TH17(23)-polarizing conditions. Our data showed that expression of only a small subset (11 out of 188 genes) of common TH17 genes, induced in both TH17(β) and TH17(23) cells, such as Il17a, Il17f and Rorc, was significantly reduced by the loss of JunB, specifically under TH17(23)-polarizing conditions (Fig. 3a and Supplementary Fig. 5a). Moreover, JunB deficiency also reduced expression of 28 of the common TH17 genes, such as Rora, Il24 and Ccr5, under both TH17(β) and TH17(23) conditions (Fig. 3a). In addition to aberrant induction of Foxp3 in TH17(β) cells, JunB deficiency also resulted in dysregulation of expression of the TH1 master transcription factor, Tbx21 (Fig. 3b), which is consistent with the reported JunB-dependent suppression of Tbx21 induction in TH2 cells40,41. qRT–PCR data confirmed the defective induction of TH17 signature genes Il17a, Il17f, Il23r and Rorc mRNAs in Junb-deficient CD4+ T cells under TH17(23)-polarizing conditions (Fig. 3c). On the other hand, JunB deficiency resulted in a significant increase in Foxp3, but it had only minor effects, if any, on expression of Il17a, Il17f, Rorc and Il23r under TH17(β) or TH17(β3) conditions (Fig. 3c and Supplementary Fig. 5b,c).
(a,b) Microarray analysis of Cd4CreJunbfl/fl and control cells activated under TH0-, TH17(β)- or TH17(23)-polarizing conditions for 60 h. Heat map data show fold changes of expression in Cd4CreJunbfl/fl (KO) versus (Junbfl/fl (control)) cells for common TH17 genes (a), and genes categorized as transcription factors (b). Only genes that showed a significant change in Cd4CreJunbfl/fl cells compared to controls are shown. Common TH17 genes represent genes significantly upregulated in both TH17(β) and TH17(23) compared to TH0 (as in Supplementary Fig. 5a). (c) Cd4CreJunbfl/fl and control cells were activated under TH17(β)- or TH17(23)-polarizing conditions for 84 h, and mRNA expression of Il17a, Il17f, Il23r and Rorc was analysed by qRT–PCR. Results were normalized to Hprt mRNA. Error bars indicate s.d. Asterisks indicate significant differences (P<0.05) by unpaired two-tailed Student’s t-test. NS, not significant. (a,b) Data represent two independent experiments. (c) Data represent three independent experiments.
We further investigated effects of JunB deficiency on gene expression profiles in IL-17A-high and IL-17-low populations induced under TH17(β) conditions. We found that JunB deficiency decreased expression of a subset of common TH17 genes, such as Ccr5 in both IL-17-high and IL-17-low populations in a similar manner (Supplementary Fig. 5d,e). However, expression of Foxp3 was significantly higher in the IL-17-low population than the IL-17-high population (Supplementary Fig. 5e), suggesting that Foxp3 may suppress IL-17 expression at the transcriptional level.
To examine whether the impairment of RORγt induction is due to aberrant induction of T-bet in Junb-deficient cells under TH17(23) conditions, we analysed RORγt and T-bet expression at the single-cell level by flow cytometry. The data showed that RORγt expression was significantly reduced not only in the T-bet-high population, but also in the T-bet-low population in Junb-deficient cells under TH17(23) conditions (Supplementary Fig. 5f), suggesting that JunB regulates expression of RORγt and T-bet independently. Collectively, these results suggest that JunB regulates a limited subset of TH17 genes in a context-dependent manner, and that activation of the RORγt-dependent core TH17 transcriptional programme relies on JunB in IL-23-dependent pathogenic TH17 cells, but not in TGF-β1-dependent non-pathogenic TH17 cells.
JunB regulates DNA binding of BATF and IRF4
To gain further insight into JunB-dependent transcriptional regulation in TH17 cells, we investigated genome-wide JunB-DNA binding in TH17 cells using chromatin immunoprecipitation sequencing (ChIP-seq) analysis with anti-JunB antibody. Consistent with the reported interaction between JunB and BATF31,33,34, we found that JunB co-localized with BATF and IRF4 at loci of not only TH17 signature genes, including Rorc, Il17a and Il23r, but also Tbx21, under both TH17(β) and TH17(23) conditions in a similar manner (Fig. 4a,b and Supplementary Fig. 6a,b), suggesting that JunB may directly regulate transcription of these genes. JunB, BATF and IRF4 were also enriched at loci of genes, induction of which was independent of JunB, such as Ctla4 (Fig. 4c and Supplementary Fig. 5b). A non-pathogenic signature cytokine gene, Il10, expression of which is regulated by BATF and IRF4 (ref. 33), was also induced in a JunB-independent manner in TH17(β) cells (Supplementary Fig. 5b). However, at the Il10 locus, there was significant enrichment of BATF, IRF4 and JunB in both TH17(β) and TH17(23) cells (Supplementary Fig. 6c), which implies that JunB co-localizes with BATF and IRF4 at a large number of gene loci, but that a limited subset of the genes is regulated by JunB.
(a–c) Naive CD4+ T cells from wild-type mice were activated under TH17(β)- or TH17(23)-polarizing conditions for 60 h and subjected to ChIP-seq analysis using JunB, BATF and IRF4 antibodies. Rorc (a), Il17a (b) and Ctla4 (c) loci are shown. Schematic representations at the tops of panels indicate transcription start sites (arrows), exons (filled boxes), introns (solid lines) and non-coding regions (dashed lines). (d–f) Naive CD4+ T cells from Cd4CreJunbfl/fl or control mice were activated under TH17(23)-polarizing conditions for 84 h and subjected to ChIP analysis using BATF, IRF4 and STAT3 antibodies. Eluted DNA was analysed by qPCR using primers to detect gene regions of Rorc (d), Il17a (e) and Ctla4 (f). Schematic representations show regions detected by PCR (open boxes). Error bars indicate s.d. Asterisks indicate significant differences (P<0.05) by unpaired two-tailed Student’s t-test. NS, not significant. Data represent two independent experiments.
Blimp1 promotes pathogenic TH17 generation, whereas CD5L is associated with non-pathogenic TH17 differentiation42,43. Although we could not detect induction of these molecules under our TH17-polarizing conditions, we found that JunB, together with BATF and IRF4, was enriched at the Prdm1 (encoding Blimp1) locus in both TH17(β) and TH17(23) cells (Supplementary Fig. 6d). Furthermore, DNA binding of JunB, BATF and IRF4 at the Cd5l locus was observed specifically in TH17(23) cells (Supplementary Fig. 6e). These findings imply that JunB might also be involved in control of these regulators of TH17 pathogenicity, probably at later stages of TH17 differentiation.
We next evaluated the impact of JunB on DNA binding of BATF, IRF4 and STAT3 during TH17 differentiation, using ChIP–PCR analysis. Loss of JunB considerably diminished DNA binding of BATF at the Rorc locus, under TH17(23) conditions, but not under TH17(β) conditions (Fig. 4d). JunB deficiency also resulted in a great reduction of binding of IRF4 and STAT3 at the Rorc locus under TH17(23) conditions, but the effect was relatively small under TH17(β) conditions (Fig. 4d). JunB deficiency also impaired binding of BATF, IRF4 and STAT3 to the Il17a locus under both TH17(β) and TH17(23) conditions (Fig. 4e), which is consistent with the positive role of JunB in IL-17A production, even under TH17(β) conditions. However, JunB deficiency did not affect the DNA binding of BATF, even though it only slightly decreased that of IRF4 and STAT3, at the Ctla4 locus under TH17(23)-polarizing conditions (Fig. 4f). Collectively, these data suggest that JunB may be critical for DNA binding of BATF, IRF4 and STAT3 at the Rorc locus in IL-23-dependent TH17 cells, but not in TGF-β1-dependent non-pathogenic TH17 cells.
JunB-independent generation of gut-resident TH17 cells
We next evaluated the importance of JunB for in vivo TH17 differentiation. We found that a substantial proportion (10–20%) of CD4+ T cells expressed IL-17A in the small intestinal lamina propria (SI LP) in Cd4CreJunbfl/fl mice, comparable to control (Junbfl/fl) mice (Fig. 5a and Supplementary Fig. 7a). Furthermore, a proportion of RORγt-expressing CD4+ T cells was similar in Cd4CreJunbfl/fl and control mice (Fig. 5b), suggesting that SI LP TH17 cells are more likely to be generated in a JunB-independent manner. Contrary to our in vitro data, showing upregulation of Foxp3 in Junb-deficient TH17(β) cells, loss of JunB did not affect the abundance of CD4+Foxp3+RORγt+ cells in SI LP (Fig. 5b), suggesting that Foxp3 induction may be suppressed in a JunB-independent manner or that Foxp3 may not be efficiently induced in gut-resident TH17 cells. Furthermore, the abundance of IL-17+CD4+ T cells in the LNs and spleens at steady state was also comparable in Cd4CreJunbfl/fl and control mice (Supplementary Fig. 7b). Thus, JunB is likely dispensable for generation of a subset of TH17 cells residing in the SI LP or peripheral lymphoid organs at steady state.
(a,b) Frequencies of CD4+ cells expressing IL-17A and IFN-γ (a) or RORγt and Foxp3 (b) were analysed by flow cytometry (n=4). Horizontal bars indicate the means. Asterisks indicate significant differences (P<0.05) by unpaired two-tailed Student’s t-test. SI LP, small intestine lamina propria. NS, not significant. Data represent two independent experiments.
JunB is required for pathogenic TH17 generation in vivo
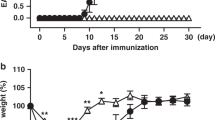
To determine the in vivo function of JunB in pathogenicity of TH17 cells, we used an EAE model, in which IL-23-dependent TH17 cells have a central pathogenic function17,18. We immunized Cd4CreJunbfl/fl mice with myelin oligodendrocyte glycoprotein (MOG)35–55 peptide in complete Freund’s adjuvant. In contrast to control mice, which developed severe EAE, Cd4CreJunbfl/fl mice were completely resistant to the disease (Fig. 6a). The number of CD4+ T cells infiltrated into the central nervous system (CNS) was much lower in Cd4CreJunbfl/fl mice than in control mice (Fig. 6b and Supplementary Fig. 7c). Furthermore, in the Cd4CreJunbfl/fl mice, there were almost no IL-17A-expressing CD4+ T cells and few IFN-γ-expressing CD4+ T cells in the CNS (Fig. 6c and Supplementary Fig. 7c). In addition, loss of JunB severely impaired production of IL-17A-expressing CD4+ T cells in LNs and spleens on day 14 after immunization (Fig. 6c and Supplementary Fig. 7d). Thus, Junb-deficient T cells are incapable of inducing EAE.
(a–c) EAE was induced in Cd4CreJunbfl/fl and control Junbfl/fl mice by immunizing with MOG peptides. (a) Disease development was monitored (n=6). Error bars indicate s.e.m. (b,c) Frequencies of CD4+ cells infiltrated in the CNS (b) and CD4+ cells expressing IL-17A and IFN-γ in the CNS, spleens and LNs (c) were analysed 14 days after EAE induction. (d,e) Il17aCreR26ReYFPJunbfl/fl or control Il17aCreR26ReYFPJunb+/+mice were immunized with MOG peptides and cells were analysed by flow cytometry on day 14 after immunization. (d) Absolute numbers of CD4+eYFP+ cells in LNs and CNS. (e) Frequencies of CD4+eYFP+ cells expressing IL-17A and IFN-γ in LNs. (f,g) Colitis was induced in Rag1-deficient mice by transferring CD4+CD45RBhiCD25− cells from Cd4CreJunbfl/fl (n=8) and control Junbfl/fl mice (n=12). (f) Body weights of mice were monitored. Error bars indicate s.e.m. (g) Frequency of CD4+ cells in the colon 28 days after transfer. Asterisks indicate significant differences (P<0.05) by unpaired two-tailed Student’s t-test. NS, not significant. (a–c) Data represent three independent experiments. (d–g) Data represent two independent experiments.
To explore the function of JunB in TH17 plasticity and stability, we performed TH17-fate-mapping analysis. We induced EAE in Junb-deficient TH17-fate-mapping reporter (Il17acreR26ReYFPJunbfl/fl) mice, in which constitutive eYFP expression and JunB deficiency are induced in cells expressing IL-17A. Our data showed that abundance of eYFP+CD4+ T cells in the LNs and CNS in Junb-deficient reporter mice was significantly lower than in controls (Il17acreR26ReYFPJunb+/+) on day 14 after immunization with MOG35–55 peptide (Fig. 6d and Supplementary Fig. 7e). Furthermore, in the eYFP+CD4+ T-cell population, Junb deficiency significantly reduced IL-17A/IFN-γ double-producing cells and IFN-γ single-producing cells, but had almost no effect on IL-17A single-producing cells (Fig. 6e), suggesting that defects in generation of TH17 cells observed in Junb-deficient mice might not be due to increased plasticity of TH17 cells. Rather, in an inflammatory context, JunB is likely required for generation of TH17 cells that are competent to differentiate to IFN-γ-producing cells.
To assess whether JunB is also required for other TH17-mediated diseases, we investigated the function of JunB in colitis development by transferring Junb-deficient CD4+CD45RBhiCD25− T cells into Rag1-deficient mice. The transfer of control, but not Junb-deficient CD4+ T cells, induced severe weight loss, beginning about 2 weeks after the transfer (Fig. 6f). Moreover, in the colonic LP of recipient mice, the frequency of IL-17A-expressing cells in the transferred Cd4CreJunbfl/fl T cells was significantly lower than of controls (Fig. 6g and Supplementary Fig. 8a). However, a substantial number of the transferred Junb-deficient CD4+ T cells expressed IFN-γ in LNs of recipient mice (Supplementary Fig. 8b).
We also investigated the function of JunB in an anti-CD3 antibody treatment model in which TH17 cells are generated and migrate into the gut44. Injection of anti-CD3 antibody increased CD4+ T cells expressing IL-17A and RORγt in the LP of duodenum in both Cd4CreJunbfl/fl and control mice, but the abundance of these cells was much lower in Cd4CreJunbfl/fl mice than in control mice (Supplementary Fig. 8c). This suggests that a subset of TH17 cells can be generated independently of JunB even in an inflammatory setting, although JunB is required for full development of inflammatory TH17 cells. Collectively, these results suggest that JunB may be selectively required for pathogenicity of TH17 cells in vivo.
Discussion
Recent studies have revealed functionally distinct subsets within a TH17 population, distinguishable by their pathogenicity and gene expression profiles1,2,3. TGF-β1/IL-6-induced TH17(β) cells are non-pathogenic, but IL-6/IL-1β/IL-23-induced TH17(23) cells are pathogenic13,14. Furthermore, IL-23 facilitates pathogenicity of TH17(β) cells14. Although a number of genes are differentially induced between TH17(β) and TH17(23) subsets, which probably contribute to their different pathogenicity, differentiation of both TH17(β) and TH17(23) subsets relies on a common TH17 transcription programme composed of BATF, IRF4, STAT3 and RORγt28,29,30,31,32. However, the mechanisms of RORγt induction in TGF-β1-dependent TH17 cells and IL-23-dependent TH17 cells have not been fully understood.
Here we demonstrate that induction of RORγt is differentially regulated in TGF-β1-dependent TH17 cells and IL-23-dependent TH17 cells. Following the RNAi screen of transcription factors responsible for IL-23 signalling, we found that JunB is critical for IL-23-dependent TH17 generation. Our in vitro results indicate that JunB is essential for RORγt induction in IL-23-dependent TH17 cells, but not in TGF-β1-dependent TH17 cells. Our in vivo results also show a similar selective requirement of JunB in a subset of TH17 cells. JunB is likely dispensable for generation of gut-resident non-pathogenic TH17 cells at steady state. However, JunB is required for generation of pathogenic TH17 cells in EAE and colitis models. A similar phenotype has been observed in mice deficient in IL-23 signalling15,17,19, supporting a model in which JunB facilitates IL-23-dependent pathogenic TH17 production.
JunB is critical for DNA binding of BATF, IRF4 and STAT3 at the Rorc locus under TH17(23)-polarizing conditions, but not under TH17(β)-polarizing conditions. However, loss of JunB has little effect on DNA binding of these transcription factors at the Ctla4 locus, expression of which is regulated independently of JunB even in TH17(23) conditions. These results suggest that JunB is likely required for recruitment or stable DNA binding of BATF, IRF4 and STAT3 in a target site-dependent manner under TH17(23)-polarizing conditions. A heterodimer of BATF and JunB, in conjunction with IRF4, binds to AP-1-IRF composite elements in many genes, including Rorc and Il17a (refs 33, 34). However, other Jun proteins, c-Jun and JunD, also interact with BATF/IRF4 and bind to AP-1-IRF composite elements33,34. Furthermore, RNAi ablation of c-Jun impairs TGF-β1-dependent TH17 differentiation45. These findings suggest that DNA-binding activity of BATF may be controlled by its AP-1 heterodimer partners, including JunB and c-Jun, in a target site- and context-dependent manner.
IL-23 is required for maturation and maintenance of TH17 cells, which are likely linked to acquisition of TH17 pathogenic cytokine-producing ability and functional plasticity in the late phase of TH17 development6. Our data show that a subset of TH17 cells is generated independently of JunB in the early phase of EAE, but full development of inflammatory TH17 cells depends on JunB. This suggests that JunB may be important for IL-23-dependent maturation and maintenance of TH17 cells, which are probably generated in a TGF-β-dependent manner. Consistent with this, our in vitro data show that JunB facilitates IL-23-dependent maintenance of IL-17-producing ability of TGF-β/IL-6-induced TH17 cells. Furthermore, in vivo TH17-fate-mapping data indicate that JunB is important for generation of TH17-derived IFN-γ-producing cells. Seemingly contradicting the in vivo observation, however, JunB deficiency results in abnormal induction of T-bet and IFN-γ in TH17 cells in vitro. In addition, our ChIP-seq results show that JunB, together with BATF, IRF4 and STAT3, binds to the Tbx21 locus, suggesting direct regulation of T-bet expression by JunB in TH17 cells. Collectively, these data suggest that JunB may control TH17 plasticity in part by positively or negatively regulating T-bet expression in TH17 cells in a developmental-phase-dependent manner, probably by interacting with distinct transcription factors or epigenetic regulators.
RORγt regulates induction of a restricted subset of TH17 genes29. Consistent with this, JunB deficiency impairs expression of a small number of genes, such as Il17a, Il17f and Il23r under TH17(23)-polarizing conditions. Despite impaired induction of a limited number of TH17 genes, Junb-deficient T cells lose their ability to induce EAE and colitis, implying a critical function of these JunB-regulated genes in TH17 pathogenicity. As Il-23r-deficient T cells are also incapable of inducing TH17-dependent diseases17, JunB-dependent IL-23R induction is likely critical for pathogenicity of TH17 cells. Furthermore, given that IL-23 signalling is important for RORγt expression in the absence of TGF-β1, it is possible that the diminished RORγt expression in Junb-deficient TH17(23) cells might be due to impaired IL-23R induction, which is needed for full induction of RORγt.
It has been suggested that GM-CSF and IFN-γ are involved in TH17 pathogenicity26,27,46. However, JunB is not required for induction of GM-CSF and IFN-γ under TH17(23)-polarizing conditions. These data suggest that GM-CSF and IFN-γ need to be produced by JunB-dependent TH17 cells to exert their pathogenic functions. A recent report demonstrated that Blimp1, which is induced by IL-23 in vivo, is critical for pathogenic TH17 differentiation42. Interestingly, our ChIP-seq data showed that JunB and BATF/IRF4 bind to the Prdm1 locus in TH17 cells in vitro, suggesting that JunB may have a function in regulation of Blimp1 expression at late stages of TH17 differentiation. We also found that JunB binds to the Cd5l locus in TH17(23) cells, but not in TH17(β) cells. CD5L is induced in non-pathogenic TH17 cells and inhibits their pathogenicity43. It is intriguing to speculate that JunB-dependent negative regulation of CD5L production may be involved in regulation of pathogenicity of TH17 cells.
Although JunB is required for RORγt induction specifically in TH17(23) cells, another pathway that is activated by TGF-β1 may compensate for the absence of JunB. Although the main TGF-β1 signalling pathway is mediated by SMAD2, SMAD3 and SMAD4 transcription factors, previous data have shown that the SMAD-dependent pathway is unnecessary for TGF-β1-dependent RORγt induction47. Our preliminary analysis showed that TGF-β1 receptor kinase inhibitor (SB43152) treatment significantly inhibited Rorc induction in TH17(β) cells, whereas there was only a partial or no reduction of Rorc expression in TH17(β) cells treated with TGF-β1 signalling regulators, including JNK inhibitor (SP600125), MEK inhibitor (PD98059), p38 inhibitor (SB203580), PI3 kinase inhibitor (LY294002), SMAD3 inhibitor (SIS3) or ROCK inhibitor (Y27632) (Supplementary Fig. 9). Thus, TGF-β receptor kinase activity may be important for JunB-independent TH17 differentiation, but the downstream signalling pathways remain unknown.
In conclusion, we demonstrate that JunB is selectively required for activation of the TH17 core transcription programme in IL-23-dependent pathogenic TH17 cells, but that the JunB-independent pathway is sufficient to activate the same programme in TGF-β1-dependent, non-pathogenic TH17 cells. JunB is essential for expression of RORγt, by promoting DNA binding of BATF, IRF4 and STAT3 at the Rorc locus in IL-23-dependent pathogenic TH17 cells. Junb-deficient T cells are incapable of inducing EAE and colitis, but loss of JunB does not seem to affect TH17 generation in the gut at steady state. Thus, the JunB-dependent pathway could be an attractive therapeutic target to suppress pathogenicity of TH17 cells, while maintaining beneficial TH17 populations.
Methods
Mice
To generate T-cell-specific, Junb-conditional knockout mice, we used C57BL/6-background ES cells, EGR-101, carrying a ‘knockout first’ Junb tm1a allele (Eucomm)48, which contains flippase recombination target (FRT)-flanked lacZ and neomycin resistance (neo) cassettes in front of a loxP-flanked (floxed) Junb exon 1 (Supplementary Fig. 2a). ES cells were injected into eight-cell Institute for Cancer Research (ICR) mouse embryos, and chimeric blastocysts were transferred into the uteri of pseudo-pregnant ICR female mice49. The resultant chimeric mice were crossed with FLP mice (Jackson, Stock No. 009086) to excise the FRT-flanked region, which generated mice carrying a conditional Junb tm1c allele (floxed Junb mice) (Supplementary Fig. 2a). Floxed Junb mice were crossed with Cd4Cre mice (Jackson) to create Cd4CreJunbfl/fl mice in which T cells carry deleted Junb (tm1d) alleles (Supplementary Fig. 2a). Il17acre (Stock No. 016879) and Rosa26eYFP (R26ReYFP) (Stock No. 006148) mice were from the Jackson Laboratory. C57BL/6 mice and floxed Stat3 mice36 were purchased from CLEA Japan and Oriental Bioservice, respectively. All mice were housed under specific pathogen-free conditions. Gender-matched 6–12-week old mice were used for experiments. All animal experiments were performed following protocols approved by Animal Care and Use Committee at Okinawa Institute of Science and Technology Graduate University.
Antibodies
The following antibodies were used for flow cytometry analysis and fluorescence-activated cell sorting (FACS): Anti-CD3 (17A2, Biolegend, 1:400), anti-CD4 (GK1.5, Biolegend, 1:100 or 1:400), anti-CD8 (53–6.7, Biolegend, 1:400), anti-CD25 (PC61, Biolegend, 1:400), anti-CD44 (IM7, Biolegend, 1:400), anti-CD62L (MEL-14, Biolegend, 1:400), anti-IL-17A (TC11-18H10.1, Biolegend, 1:100), anti-IFN-γ (XMG1.2, Biolegend, 1:100), anti-GM-CSF (MP1-22E9, BD, 1:100), anti-RORγt (Q31-378, BD, 1:50), anti-Foxp3 (150D, Biolegend, 1:50), anti-T-bet (4B10, Biolegend, 1:50) and anti-TCRVβ8.1/8.2 (KJ16-133.18, Biolegend, 1:20). For immunoblot analyses, anti-JunB (C37F9, Cell Signaling Technology, 1:2,000), anti-RORγt (AFKJS-9, eBioscience, 1:400) and anti-GAPDH (3H12, MBL, 1:2,000) were used. For ChIP analyses, anti-JunB (210, Santa Cruz, 2 μg per ChIP), anti-BATF (WW8, Santa Cruz, 2 μg per ChIP), anti-IRF4 (M-17, Santa Cruz, 2 μg per ChIP), anti-STAT3 (c-20, Santa Cruz, 2 μg per ChIP) were used.
In vitro CD4+ T-cell differentiation
CD4+ T cells from single-cell suspensions of murine spleens and LNs were enriched using a MACS magnetic cell sorting system with anti-CD4 microbeads (130-049-201, Miltenyi). Then naive CD4+ T cells (CD4+CD25-CD62LhiCD44lo) were sorted by FACS AriaII or AriaIII (BD). Cells were activated with plate-bound anti-CD3 antibody (5 μg ml−1; 145-2C11, Biolegend) and soluble anti-CD28 antibody (1 μg ml−1, 37.51, Biolegend) in IMDM media (Invitrogen) supplemented with 10% foetal calf serum (Invitrogen) containing the following cytokines and antibodies: IL-2 (20 ng ml−1; Biolegend), anti-IFN-γ (1 μg ml−1; Biolegend) and anti-IL-4 (1 μg ml−1; Biolegend) for TH0; IL-6 (20 ng ml−1; Biolegend) and TGF-β1 (3 ng ml−1; Miltenyi) for TH17(β); IL-6 (20 ng ml−1; Biolegend), IL-1β (20 ng ml−1; Bioelegend) and IL-23 (40 ng ml−1; Biolegend) for TH17(23); IL-6 (20 ng ml−1) and TGF-β3 (3 ng ml−1; Miltenyi) for TH17(β3);TGF-β1 (15 ng ml−1) and IL-2 (20 ng ml−1) for iTreg differentiation. For analysis of cytokine expression, cells were re-stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng ml−1; Sigma) and ionomycin (500 ng ml−1; Sigma) in the presence of brefeldin A (5 μg ml−1; Biolegend) for 4 h. Then, cells were fixed with 4% paraformaldehyde, permeabilized in permeabilization/wash buffer (421002, Biolegend), and stained with antibodies against cytokines. For analysis of expression of RORγt and Foxp3, Foxp3 staining buffer set (00-5253-00, eBioscience) was used according to the instructions. In some experiments, TGF-β1 receptor kinase inhibitor (SB43152, 10 μM; Selleckchem), JNK inhibitor (SP600125, 10 μM; EMD Millipore), MEK inhibitor (PD98059, 10 μM; Invivogen), p38 inhibitor (SB203580, 10 μM; Invivogen), PI3 kinase inhibitor (LY294002, 5 μM; Invivogen), SMAD3 inhibitor (SIS3, 10 μM; Sigma) or ROCK inhibitor (Y27632, 10 μM; Sigma) were added to the culture media. Dead cells were excluded using Zombie NIR Fixable viability kits (423106, Biolegend) for flow cytometry analysis. All flow cytometry gating strategies are shown in Supplementary Fig. 10.
Cell isolation
Mononuclear cells were isolated from SI LP or colonic LP using Lamina Propria Dissociation kits (130-397-410, Miltenyi). For isolation of cells from CNS of EAE-induced mice, brains and spinal cords were digested with collagenase D (1 mg ml−1; Roche) and DNase I (2.5 mg ml−1; Sigma) in phosphate-buffered saline at 37 °C for 1 h with shaking. Then, isolated cells were re-suspended in 37% Percoll (GE Healthcare), mixed with 70% Percoll and then centrifuged for 20 min. Mononuclear cells were isolated from the interface. Cells were incubated with anti-Fc receptor blocking antibody (anti-CD16/CD32; Biolegend) and then stained with antibodies against cell surface molecules. Expression of cytokines and transcription factors was analysed by flow cytometry as described above.
Enrichment of IL-17-secreting cells
We enriched IL-17-secreting cells with a mouse IL-17 secretion assay kit (Miltenyi), according to the manufacturer’s instructions. Briefly, naive CD4 T cells were activated in the presence of TGF-β1 and IL-6, as above. On day 3, cells were re-stimulated with PMA (10 ng ml−1) and ionomycin (1 μg ml−1) for 3 h, followed by IL-17-capture reaction in serum-free media (X-VIVO20; Lonza). Phycoerythrin-labelled IL-17-secreting cells were sorted using a FACS AriaIII.
Retrovirus infection
The pMKO1.GFP retroviral vector (a gift from William Hahn, Addgene plasmid # 10676), which is a bicistronic vector containing an internal ribosome entry site (IRES). IRES-driven complementary DNA (cDNA) encoding green fluorescent protein (GFP) was used for shRNA transduction. Briefly, PlatE cells were transfected with pMKO1.GFP containing shRNAs and the pCL-Eco helper plasmid using polyethylenimine. pCL-Eco was a gift from Inder Verma (Addgene plasmid # 12371)50. Culture supernatants were collected at 72 h post transfection, supplemented with polybrene (8 μg ml−1), and added to sorted naive CD4+ T cells (2 × 105 cells per well in a 48-well plate) previously stimulated for 36 h under TH0-polarization conditions. Cultures were centrifuged at 300g for 60 min at room temperature, and media was replaced with fresh media containing TH17(23) cytokines. Cells were incubated for 3 days, and GFP+ cells were sorted for RNA isolation and qRT–PCR. Sequences of shRNAs against 263 transcription factors highly expressed in TH17 cells are listed in Supplementary Data 1.
qRT–PCR
Total RNA isolated from cells using an RNeasy Plus Mini Kit (74136, Qiagen) was used for cDNA synthesis with a Revertra Ace qPCR Kit (FSQ-101, Toyobo). The resulting cDNA was used as a template for qRT–PCR performed with Faststart SYBR master mix (4673484, Roche) and a Thermal Cycler Dice Real Time system (Takara). Primers used for qPCR are listed in Supplementary Table 1.
Immunoblot analysis
Cells were lysed with RIPA buffer (Thermo) containing complete protease inhibitor cocktail (4693159, Roche). Cellular debris in the lysate was removed by centrifugation at 14,000g for 15 min. The protein concentration was measured with a DC Protein Assay kit (500-0106, Bio-Rad). Protein extracts were mixed with 5 × sample loading buffer (250 mM Tris-HCl pH 6.8, 10% SDS, 30% glycerol, 5% β-mercaptoethanol and 0.02% bromophenol blue) and subjected to SDS–polyacrylamide gel electrophoresis. Then separated proteins on the gel were transferred onto Immobilon P transfer membranes (Millipore) using a Trans-blot electrophoretic transfer system (Bio-Rad). Membranes were blocked with 5% skim milk (Wako) in phosphate-buffered saline with 0.1% Tween-20 (Sigma) and incubated with the indicated primary antibodies and probed with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibodies (Cell Signaling Technology, 1:4,000). Bound antibody was detected with Clarity Western ECL (Bio-Rad) or SuperSignal West Femto detection reagents (Thermo) and an Las-3000 imaging system (Fuji film). Uncropped original scans of immunoblots were provided in Supplementary Fig. 11.
Microarray analysis
Total RNA was isolated from cells using an RNeasy Plus Mini Kit (74136, Qiagen). RNA quality was analysed with an Agilent 2100 Bioanalyzer and an RNA 6000 Nano Kit (5067-1511, Agilent). RNA samples showing RNA integrity numbers ≥7 were used to generate biotinylated complementary RNA (cRNA) with a GeneChIP WT Plus Reagent Kit (902280, Affymetrix) and GeneChip Hybridization, Wash and Stain Kit (900720, Affymetrix). Labelled cRNA was hybridized to Affymetrix Mouse Gene 1.0 ST microarrays (Affymetrix), then stained and scanned with an Affymetrix GeneChip 3000 (Affymetrix).
ChIP-seq and ChIP–PCR analysis
ChIP was performed using a SimpleChIP Plus Enzymatic Chromatin IP Kit (9005S, Cell Signaling) with some modifications, as previously described51,52. Briefly, activated T cells (106 per ChIP-seq or 1–3 × 105 per ChIP–PCR) were crosslinked in culture medium containing 1% formaldehyde at room temperature for 10 min, and the reaction was stopped by adding glycine solution. Then cells were lysed, and nuclei were collected and treated with micrococcal nuclease (0.0125 μl ml−1) for 20 min at 37 °C. After stopping the reaction with 0.05 M EGTA, samples were sonicated with several pulses to disrupt nuclear membranes. Then the supernatant, containing chromatin, was collected after centrifugation. Chromatin solutions were incubated with 2 μg of antibodies overnight at 4 °C with rotation, followed by incubation with Protein G magnetic beads for 2 h at 4 °C. Beads were washed, and chromatin was eluted. Crosslinks were reverted according to kit instructions. DNA was purified by phenol/chloroform extraction and used for ChIP–PCR analysis with primers listed in Supplementary Table 1.
For ChIP-seq analysis, sample DNA was quantified with a Qubit 3.0 Fluorometer (Thermo Fisher Scientific) and normalized to 200 pg as starting DNA for library preparation. Libraries were prepared using KAPA Hyper Prep Kit (KK8500, KAPA Biosystems) protocols for blunt-ending, polyA extension and adaptor ligation. Post-ligation clean-up with an Agencort AMPure XP (Beckman Coulter) was performed at a 1.8 × DNA ratio to purify ligated DNA, which was then PCR-amplified and purified using AMPure XP at a 1.2 × DNA ratio to remove excess adaptor-dimer and to preserve small fragments. Size selection was performed with a 2% agarose gel cassette of Blue Pippin (Sage Science) for a target insert size between 30 and 180 bp. Library quantification was performed by droplet digital PCR (Bio-Rad). All libraries were pooled and loaded onto cBot (50 μl of 200 pM) for cluster generation, and then sequenced on an Illumina HiSeq4000 at a target sequencing depth of 10 million uniquely aligned reads.
EAE induction
Six–eight-week old, gender-matched mice were immunized with MOG35–55 peptides (300 μg per mouse) in complete Freund's adjuvant (CFA) (100 μl per mouse) containing dead Mycobacterium tuberculosis (1 mg per mouse). Pertussis toxin (400 ng per mouse) was also intraperitoneally injected into the mice twice on day 0 and on day 2 post immunization. Disease severity was evaluated on a scale of 1–5 as follows: 1, limp tail; 2, partially paralysed hind legs; 3, completely paralysed hind legs; 4, complete hind and partial front leg paralysis; 5, completely paralysed hind and front legs. Mice with disease score 5 were considered moribund and were killed by CO2 inhalation.
Colitis induction
CD4+CD45RBhiCD25− T cells were purified from CD4+ T cells isolated from spleens and LNs of Cd4CreJunbfl/fl and control mice, and intraperitoneally injected into Rag1-deficient mice (4 × 105 cells per mouse). Disease progress was monitored by weighing the mice.
Anti-CD3 antibody treatment
We injected mice with anti-CD3 antibody (50 μg per mouse) three times at 0, 48 and 96 h. At 4 h after the final injection, the mice were killed by CO2 inhalation for cell isolation from LP of duodenums.
Statistical analysis
Statistical analyses were performed using unpaired two-tailed Student’s t-test with Prism software (GraphPad). P values <0.05 were considered as significant.
Data availability
Microarray and ChIP-seq data that support the findings of this study have been deposited in the Gene Expression Omnibus with the primary accession codes GSE86499 and GSE86535, respectively. The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary Information files or are available from the authors on request.
Additional information
How to cite this article: Hasan, Z. et al. JunB is essential for IL-23-dependent pathogenicity of Th17 cells. Nat. Commun. 8, 15628 doi: 10.1038/ncomms15628 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Murphy, K. M. & Stockinger, B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat. Immunol. 11, 674–680 (2010).
Ghoreschi, K., Laurence, A., Yang, X. P., Hirahara, K. & O'Shea, J. J. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 32, 395–401 (2011).
Patel, D. D. & Kuchroo, V. K. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity 43, 1040–1051 (2015).
Lai, Y. & Dong, C. Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases. Int. Immunol. 28, 181–188 (2016).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Honda, K. & Littman, D. R. The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016).
Atarashi, K. et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 (2008).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003).
Mangan, P. R. et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441, 231–234 (2006).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Ghoreschi, K. et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467, 967–971 (2010).
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
McGeachy, M. J. et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
McGeachy, M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Haines, C. J. et al. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Rep. 3, 1378–1388 (2013).
Ahern, P. P. et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33, 279–288 (2010).
Hirota, K. et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12, 255–263 (2011).
Hirota, K. et al. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 14, 372–379 (2013).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Yosef, N. et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature 496, 461–468 (2013).
Wu, C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013).
Gaublomme, J. T. et al. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 163, 1400–1412 (2015).
Codarri, L. et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011).
El-Behi, M. et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011).
Ivanov, I. I. et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012).
Brustle, A. et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 8, 958–966 (2007).
Schraml, B. U. et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature 460, 405–409 (2009).
Yang, X. O. et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282, 9358–9363 (2007).
Li, P. et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature 490, 543–546 (2012).
Glasmacher, E. et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science 338, 975–980 (2012).
Zhong, Z., Wen, Z. & Darnell, J. E. Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264, 95–98 (1994).
Takeda, K. et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161, 4652–4660 (1998).
Morikawa, H. & Sakaguchi, S. Genetic and epigenetic basis of Treg cell development and function: from a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol. Rev. 259, 192–205 (2014).
Zhou, L. et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453, 236–240 (2008).
Ichiyama, K. et al. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J. Biol. Chem. 283, 17003–17008 (2008).
Li, B., Tournier, C., Davis, R. J. & Flavell, R. A. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J. 18, 420–432 (1999).
Hartenstein, B. et al. Th2 cell-specific cytokine expression and allergen-induced airway inflammation depend on JunB. EMBO J. 21, 6321–6329 (2002).
Jain, R. et al. Interleukin-23-induced transcription factor Blimp-1 promotes pathogenicity of T helper 17 cells. Immunity 44, 131–142 (2016).
Wang, C. et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163, 1413–1427 (2015).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Ichiyama, K. et al. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-beta is mediated by suppression of eomesodermin. Immunity 34, 741–754 (2011).
Wang, Y. et al. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-gamma-producing T helper 17 cells. Immunity 40, 355–366 (2014).
Takimoto, T. et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J. Immunol. 185, 842–855 (2010).
Skarnes, W. C. et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474, 337–342 (2011).
Fujihara, Y., Kaseda, K., Inoue, N., Ikawa, M. & Okabe, M. Production of mouse pups from germline transmission-failed knockout chimeras. Transgenic Res. 22, 195–200 (2013).
Naviaux, R. K., Costanzi, E., Haas, M. & Verma, I. M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70, 5701–5705 (1996).
Tsankov, A. M. et al. Transcription factor binding dynamics during human ES cell differentiation. Nature 518, 344–349 (2015).
Henikoff, J. G., Belsky, J. A., Krassovsky, K., MacAlpine, D. M. & Henikoff, S. Epigenome characterization at single base-pair resolution. Proc. Natl Acad. Sci. USA 108, 18318–18323 (2011).
Acknowledgements
We thank M. Matmati, B. Parajuri, S. Nishijima, H. Goto and M. Igawa for discussion and assistance, S. Akira for floxed Stat3 mice, C. Wilson for Cd4Cre mice, W. Haln and I. Verma for plasmid constructs and S. Aird for editing the manuscript. We also thank NPO Biotechnology Research and Development for technical assistance. We are also grateful to OIST Graduate University for its generous funding of the Immune Signal Unit.
Author information
Authors and Affiliations
Contributions
Z.H., S.-i.K., D.S., H.Y., S.O. and H.S. conducted experiments. Z.H., S.-i.K., and D.S. analysed data. N.A. carried out ChIP-seq experiments. Y.F. generated floxed JunB mice. H.I. designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures and Supplementary Table 1 (PDF 1229 kb)
Supplementary Data 1
shRNA sequences. (XLSX 27 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hasan, Z., Koizumi, Si., Sasaki, D. et al. JunB is essential for IL-23-dependent pathogenicity of Th17 cells. Nat Commun 8, 15628 (2017). https://doi.org/10.1038/ncomms15628
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms15628
This article is cited by
-
Depletion of JunB increases adipocyte thermogenic capacity and ameliorates diet-induced insulin resistance
Nature Metabolism (2024)
-
Dysregulation of Wnt/β-catenin signaling contributes to intestinal inflammation through regulation of group 3 innate lymphoid cells
Nature Communications (2024)
-
JAKs and STATs from a Clinical Perspective: Loss-of-Function Mutations, Gain-of-Function Mutations, and Their Multidimensional Consequences
Journal of Clinical Immunology (2023)
-
Morin, the PPARγ agonist, inhibits Th17 differentiation by limiting fatty acid synthesis in collagen-induced arthritis
Cell Biology and Toxicology (2023)
-
Bergenin, a PPARγ agonist, inhibits Th17 differentiation and subsequent neutrophilic asthma by preventing GLS1-dependent glutaminolysis
Acta Pharmacologica Sinica (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.