Abstract
Lung function measures are used in the diagnosis of chronic obstructive pulmonary disease. In 38,199 European ancestry individuals, we studied genome-wide association of forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC with 1000 Genomes Project (phase 1)-imputed genotypes and followed up top associations in 54,550 Europeans. We identify 14 novel loci (P<5 × 10−8) in or near ENSA, RNU5F-1, KCNS3, AK097794, ASTN2, LHX3, CCDC91, TBX3, TRIP11, RIN3, TEKT5, LTBP4, MN1 and AP1S2, and two novel signals at known loci NPNT and GPR126, providing a basis for new understanding of the genetic determinants of these traits and pulmonary diseases in which they are altered.
Similar content being viewed by others
Introduction
Lung function, as measured by spirometry, predicts morbidity and mortality1,2. Altered lung function is a key criterion for the diagnosis of chronic obstructive pulmonary disease (COPD), a leading cause of death worldwide3,4. The ratio of forced expiratory volume in 1 s (FEV1) over forced vital capacity (FVC) defines patients with airflow obstruction, while FEV1 is used to assess the severity of the obstruction. Reduced FVC values are seen in restrictive lung diseases such as pulmonary fibrosis5. While environmental risk factors, such as tobacco smoking or air pollution, play a significant role in determining lung function6,7, genetic factors are also important contributors, with estimates of heritability ranging between 39 and 54% (refs 8, 9).
Genome-wide association studies (GWAS) of around 2.5 million common (minor allele frequency (MAF)>5%) single-nucleotide polymorphisms (SNPs) in Europeans have identified 32 loci associated with lung function at genome-wide significance level (P<5 × 10−8)10,11,12,13,14. However, as for other complex traits15,16, these loci only explain a limited proportion of the heritability11,13. Among explanations for the ‘missing heritability’ are a large number of, as yet, undetected common variants with modest effect sizes, in addition to low-frequency (1%<MAF≤5%) and rare (MAF≤1%) variants with larger effect sizes16,17. Of particular relevance to low-frequency variants, phase 1 of the 1000 Genomes Project18 sequenced 1,092 individuals from 14 populations, providing an imputation reference panel of ∼38 million SNPs and 1.4 million indels, including autosomal and X chromosome variants.
The aim of the current study, undertaken within the SpiroMeta consortium, was to improve coverage of low-frequency variants and detect novel loci associated with lung function by undertaking imputation of GWAS data to the 1000 Genomes Project18 Phase-1 reference panel in 38,199 individuals of European ancestry. We meta-analysed GWAS results across 17 studies and followed up the most significant associations with in silico data in up to 54,550 Europeans. We identify 14 new loci associated with lung function at genome-wide significance level, and novel distinct signals at two previously reported loci. These include two low-frequency variant association signals, which seem to be explained by non-synonymous SNPs. The results of these analyses implicate both previously considered and novel mechanisms influencing lung function.
Results
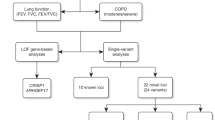
We undertook a meta-analysis of 17 GWAS imputed using the 1000 Genomes Project18 Phase-1 reference panel in a study of 38,199 individuals of European ancestry in stage 1 (Fig. 1), of which 19,532 were individuals not included in the discovery stage of previous meta-analyses of lung GWAS10,11,12,13. Characteristics of cohort participants, genotyping and imputation are shown in Supplementary Table 1. Each study adjusted FEV1, FEV1/FVC and FVC, for age, age2, sex, height and principal components for population structure, separately for never and ever smokers. Fourteen studies additionally undertook analyses for X chromosome variants (33,009 individuals, Supplementary Fig. 1 and Methods). Inverse normally transformed residuals were then used for association testing within each smoking stratum, assuming an additive genetic effect. Within each study, we combined smoking strata association summary statistics using inverse variance-weighted fixed-effects meta-analysis, and applied genomic control19 to account for residual population structure not accounted for by principal components. We subsequently combined study-specific estimates across studies using inverse variance weighing, and applied genomic control19 after fixed-effects meta-analysis. The genomic inflation factor across autosomal variants was 1.03 for each of the three traits, and across X chromosome variants was 1.04 for FEV1 and 1.00 for FEV1/FVC and FVC. Quantile–quantile plots are presented in Supplementary Fig. 2a. Variants with effective sample sizes (N effective, product of sample size and imputation quality summed across studies) <70% were filtered out, and a total of 8,694,268 variants were included in this genome-wide study.
Forty-eight SNPs and seven indels in independent autosomal chromosome regions (±500 kb either side of sentinel variant) with stage 1 P<5 × 10−6 were followed up in stage 2 using in silico data from four studies comprising 54,550 individuals (Fig. 1; Supplementary Table 2). One SNP on the X chromosome also met these criteria and was followed up in a subset of three studies comprising 52,359 individuals (Supplementary Fig. 1; Supplementary Table 2). Characteristics of follow-up (stage 2) cohort participants, genotyping and imputation are shown in Supplementary Table 1. Stage-2 studies adjusted the traits for age, age2, sex, height and principal components to account for population structure and ever-smoking status, and also undertook association testing on the inverse normally transformed residuals assuming additive genetic effects. Stage-2 estimates were combined across studies, and then with stage-1 estimates, using inverse variance-weighted fixed-effects meta-analysis. Thirteen SNPs and three indels, each representing new signals of association, met a genome-wide significance threshold corrected for multiple testing (P<5 × 10−8) after combining stage-1 and stage-2 results (Table 1; Fig. 2), of which 10 SNPs and three indels achieved independent replication meeting a Bonferroni-corrected threshold for 56 tests (P<8.93 × 10−4) in stage 2 alone.
(a) FEV1, (b) FEV1/FVC and (c) FVC. Manhattan plots ordered by chromosome and position for stage-1 results. Variants with P<5 × 10−6 are indicated in red. Novel signals that reached genome-wide significance after meta-analysing stage 1 and stage 2 are labelled with the nearest gene. Only variants with N effective ⩾70% are presented here.
Sixteen novel association signals for FEV1, FEV1/FVC and FVC
Of the 16 novel signals reaching genome-wide significance, two represent distinct new signals for FEV1/FVC in previously reported loci10,12 (stage-1 P value conditioned on previously reported variant <5 × 10−6). Among the remaining 14, five new loci were identified for FEV1, six new loci for FEV1/FVC and three new loci for FVC (Table 1). The sentinel variants at the 16 loci were in or near the following genes: MCL1-ENSA (1q21.3), LYPLAL1-RNU5F-1 (1q41), KCNS3-NT5C1B (2p24.2), AK097794 (3q25.32), NPNT (4q24), GPR126-LOC153910 (6q24.1), ASTN2 (9q33.1), LHX3 (9q33.1), PTHLH-CCDC91 (12p11.22), TBX3 (12q24.21), TRIP11 (14q32.12), RIN3 (14q32.12), EMP2-TEKT5 (16p13.13), LTBP4 (19q13.2), MIAT-MN1 (22q12.1) and on chromosome X, AP1S2-GRPR (Xp22.2) (Supplementary Fig. 2b,c). To gain further insight into the associated variants, we assessed whether the novel sentinel variants, or their proxies, were associated with gene expression in lung tissues20 and blood21 (Methods, Supplementary Methods; Supplementary Table 3a) or were in DNase hypersensitivity sites22 in relevant cell types (Methods; Supplementary Table 3b). For relevant genes, we investigated RNA-seq splice isoforms in human bronchial epithelial cells (Supplementary Methods; Supplementary Fig. 3), searched for evidence of protein expression in the respiratory system23 (Supplementary Table 3c), assessed differential expression across the pseudoglandular and canalicular stages of fetal human lung development (Methods; Supplementary Table 3d) and assessed evidence for differences in gene expression in bronchial epithelial brush samples from COPD cases and smoking controls (Methods; Supplementary Table 3e).
The two novel signals in known loci were the strongest (P<5 × 10−23) association signals after meta-analysing stage 1 and 2. The strongest signal was for a low-frequency SNP near GPR126 (rs148274477, MAF=2.4%, intergenic on chromosome 6) associated with FEV1/FVC (P=9.6 × 10−26, Table 1) and in high linkage disequilibrium (LD, r2=0.85) with a missense variant (rs17280293 (Ser123Gly), Supplementary Table 3f) in GPR126, but distinct from the previously reported signal for FEV1/FVC in this region10,13 (stage-1 P value for rs148274477 conditioning on rs3817928 (ref. 10) and rs262129 (ref. 13)=1.86 × 10−7, unconditional stage-1 P=2.68 × 10−9). GPR126 encodes a G-protein-coupled receptor and is expressed in adult and fetal lung tissue24,25 (Supplementary Table 3d). Other studies have shown that GPR126 is required for mice embryonic viability and cardiovascular development26, and that GPR126 is expressed in adult mice lung27. More recently, GPR126 has been shown to bind type-IV collagen, a major collagen in the lung, leading to cAMP signalling28.
The second strongest signal (P=1.5 × 10−23, Table 1) was an intronic SNP (rs6856422) in NPNT on chromosome 4 associated with FEV1/FVC, distinct from the previously discovered signal for FEV1 in this region10,12,13. The stage-1 P value for this variant conditioned on the previously reported sentinel SNPs (rs17036341 (ref. 10) and rs10516526 (refs 12, 13)) and on the sentinel SNP for FEV1 in this analysis (rs12374256, INTS12 intron) was 4.7 × 10−6 (unconditional stage-1 P=1.30 × 10−7). Proxies of the sentinel SNP were associated with expression of INTS12 and GSTCD in blood (Supplementary Table 3a). INTS12, GSTCD and NPNT are contiguously positioned at 4q24, and are all expressed in adult and fetal lung tissues (Supplementary Table 3c,d). Our previous work characterizing GSTCD and INTS12 showed that they are oppositely transcribed genes that are to some extent co-ordinately regulated, although while GSTCD expression in human lung tissue is ubiquitous, INTS12 expression was predominantly in the nucleus of epithelial cells and pneumocytes29.
Among the 14 novel loci, six novel loci were associated with FEV1/FVC. One of them was a low-frequency variant (rs113473882, intronic in LTBP4 on chromosome 19, MAF=1.5%, Table 1) in almost complete LD (r2=0.99) with a missense variant (rs34093919, Asp752Asn, Supplementary Table 3f) in LTBP4, which encodes a protein that binds transforming growth factor beta (TGFβ) as it is secreted and targeted to the extracellular matrix. Mice deficient in ltbp4 displayed defects in lung septation and elastogenesis, which may be TGFβ2 and fibulin-5 dependent30, and disruption of this gene in mice led to abnormal lung development, cardiomyopathy and colorectal cancer31. Variants near LTBP4, uncorrelated (r2<0.05) with the sentinel SNP we report here, have been associated with COPD32 and smoking behaviour33. A further novel FEV1/FVC locus mapping near AP1S2 is the first to be reported for lung function on the X chromosome; sentinel SNP (rs7050036, intergenic) proxies were associated with the expression of AP1S2 and ZRSR2 in lung tissue (Supplementary Table 3a). Other new loci for FEV1/FVC were in or near KCNS3 (2p24.2), ASTN2 (9q33.1), RNU5F-1 (1q41) and TEKT5 (16p13.13).
The strongest signal for FEV1 in a novel locus was upstream of TBX3 on chromosome 12 (Table 1); TBX3 is involved in the TGFβ1 signalling pathway34. At a second novel locus for FEV1 (rs7155279, TRIP11 intron on chromosome 14, Table 1), proxies of the sentinel variant were associated with lung and blood expression of TRIP11. TRIP11 encodes a protein associated with the Golgi apparatus35. In the lung, rs7155279 showed strongest association with expression of ATXN3 (Supplementary Table 3a), which encodes ataxin 3, a deubiquitinating enzyme. Expanded trinucleotide repeats in ATXN3 cause spinocerebellar ataxia-3 (ref. 36). In blood, a proxy (r2=0.94) for rs7155279 showed strong association (P=3 × 10−34, Supplementary Table 3a) with the expression of FBLN5. Fibulin-5 was shown to be implicated in tissue repair in COPD37 and elastogenesis and lung development30. A third signal for FEV1 was a missense variant (rs117068593, Arg279Cys, Supplementary Table 3f) in RIN3 on chromosome 14 (Table 1), which was ∼632 kb from the TRIP11 sentinel SNP (rs7155279) and independent from it (r2=8.84 × 10−5). Although this is the first report of association of a RIN3 variant with lung function, a correlated variant (rs754388, r2=0.99) was recently associated with moderate to severe COPD, although the association did not replicate in an independent study38. In a fourth novel region for FEV1, on chromosome 1, a sentinel SNP, rs6681426, ∼8 kb downstream of ENSA (Table 1) and a second signal ∼700 kb apart (rs4926386, Supplementary Table 2a) were both associated with ARNT expression in lung (Supplementary Table 3a). ARNT is differentially expressed during fetal lung development (Supplementary Table 3d) and acts as a co-factor for transcriptional regulation by hypoxia-inducible factor 1 during lung development39 and may regulate cytokine responses40. SNP rs6681426 was also associated with the expression of LASS2 (also known as CERS2) in lung tissue (Supplementary Table 3a); lass2 knock-out mice develop lung inflammation and airway obstruction41. The other new locus for FEV1 was near MN1 (22q12.1).
All three novel loci for FVC had sentinel variants or close proxies associated with expression of a nearby gene in lung, implicating CCDC91, MLF1 and QSOX2, located on chromosomes 12, 3 and 9, respectively. The putative function of the key genes in each of the two known and 14 novel loci for FEV1, FEV1/FVC and FVC are summarized in Supplementary Table 4.
Functional characterization of novel signals
The protein products of genes nearest to the sentinel variant of novel signals for lung function were expressed in bronchial epithelial cells, pneumocytes or lung macrophages (Supplementary Table 3c). Among the 16 novel signals of association with lung function, sentinel variants or close proxies were cis expression quantitative trait loci (eQTLs) in lung for ARNT, MLF1, QSOX2, CCDC91 and ATXN3 (Table 1; Supplementary Table 3a), and in eight loci the sentinel variant or at least one strong proxy (r2>0.8) was in a DNase hypersensitivity site in a cell type potentially relevant to lung function (in or near ENSA, RNU5F-1, ASTN2, CCDC91, TBX3, RIN3, TEKT5 and MN1, Supplementary Table 3b). The sentinel variant association was explained (conditional P>0.01) by a missense variant in each of the two novel signals in which we detected a low-frequency sentinel variant (near GPR126 and in LTBP4), and was explained in four of the remaining novel signals by a putatively functional variant (in or near ENSA, AK097794, TEKT5 and MN1, Supplementary Table 3f and Methods). Genes in four of the novel loci showed differential expression across the pseudoglandular and canalicular stages of fetal lung development, particularly EMP2 (Supplementary Table 3d). MLF1 and ATXN3 showed differences in expression levels in bronchial brushings between COPD cases and controls (Supplementary Table 3e). We detected novel splice isoforms of >20% abundance for GFM1, TRIM32, LTBP4 and MN1 in human bronchial epithelial cells (Supplementary Fig. 3; Supplementary Methods).
Association in children
To assess whether the 16 new sentinel variants associated with lung, function in adults may act through an effect on lung development, we assessed their association in the ALSPAC study42 that includes 5,062 children (Supplementary Table 5a). Eleven of the 16 sentinel variants showed consistent directions of effect in adults and children. The association with FVC of variant rs6441207 on chromosome 3 in the noncoding RNA AK097794 exceeded a Bonferroni-corrected threshold for 16 tests (Supplementary Table 5a).
Association with smoking and gene by smoking interaction
The 16 new variants had consistent effect sizes in never smokers and ever smokers, and no gene–smoking interaction (P>0.05) in stage 1 (Supplementary Table 5b). We found no evidence that any of these signals were driven by smoking behaviour. Only the two-base-pair insertion on chromosome 1 (rs201204531) revealed an association (P=1.5 × 10−3) with smoking behaviour (heavy- versus never-smoking status) that met a Bonferroni-corrected threshold for 16 tests (Supplementary Table 5c). However, this variant also showed an association with FEV1/FVC in never smokers, and the allele associated with higher likelihood of being a smoker was associated with increased FEV1/FVC (Supplementary Table 5b,c).
Associations with other traits
We queried the GWAS catalog43 for variants in 2-Mb regions centred on the sentinel variant for the 16 loci (Supplementary Table 5d). Five loci contained variants associated with height44,45,46 (Supplementary Table 5d). In the GPR126 and LHX3 loci, the previously reported height variants were not correlated (r2<0.2) with the lung function variants reported here. In the AK097794, CCDC91 and TRIP11 loci, the variants associated with height were correlated (r2>0.3) with the lung function sentinel variants, but the alleles associated with reduced height were associated with increased FEV1 or FVC. Associations with other traits have been reported for variants in LD (r2>0.3) with sentinel variants in regions of RIN3 (Paget’s disease47 and bone mineral density48), ENSA (body fat mass49 and melanoma50) and LHX3 (thyroid hormone levels51). None of the novel signals relate to known asthma loci, and the association findings were consistent after removing individuals with asthma (Supplementary Fig. 4).
Genetic architecture of lung function traits
The proportion of the additive polygenic variance explained by the 49 signals discovered to date (Supplementary Table 6), including new and previously reported signals10,11,12,13,14 is 4.0% for FEV1, 5.4% for FEV1/FVC and 3.20% for FVC (Supplementary Table 7). These estimates are likely upper bounds on the proportion of the variance explained due to the winner’s curse bias. Across the 49 signals, we observed larger effect sizes for associations with lower-frequency variants (Fig. 3), supporting the hypothesis that lower-frequency variants will contribute to explaining the missing heritability16.
(a) FEV1, (b) FEV1/FVC and (c) FVC. MAF is plotted against stage-1 effect sizes for variants within the 33 known10,11,12,13,14 and the 16 new signals, which had stage-1 P<0.05 for association with FEV1, FEV1/FVC and FVC separately. Known signals are represented with blue circles and new signals are represented with orange triangles.
We examined the increase in coverage of low-frequency and common variants by the 1000 Genomes Project reference panel, compared with the HapMap imputation reference panel, at both the novel and previously reported loci (Supplementary Fig. 5a). The two association signals where the 1000 Genomes sentinel variants had low MAF (<5%), were not present when restricting the results only to variants that could be imputed using the HapMap imputation panel (rs113473882 and rs148274477 in Supplementary Fig. 5a).
For each of the 32 previously discovered regions10,11,12,13,14, we identified the most strongly associated variant present on the 1000 Genomes Project18 reference panel and the most strongly associated variant present on the HapMap reference panel using stage-1 results, and compared the stage-1 MAFs between these two groups of variants. The 1000 Genomes sentinel variants in or near GPR126 (rs148274477), TGFB2 (rs147187942) and MMP15 (rs150232756) had MAFs that were more than twofold lower than the HapMap sentinel variant MAFs (Supplementary Fig. 5b) and were statistically independent (r2≤0.06) from the previously discovered HapMap-imputed sentinel variants13. The GPR126 1000 Genomes-imputed sentinel was described above as one of the 16 new signals. We tested the association of the 1000 Genomes-imputed sentinel variants near TGFB2 and MMP15 in UK BiLEVE (Supplementary Table 8), and found supportive evidence of association for the signal near TGFB2 (rs147187942, MAF=9%, P=5.7 × 10−3).
Pathway analyses
We undertook a pathway analysis using MAGENTA v2 (ref. 52) and stage-1 genome-wide results for FEV1, FEV1/FVC and FVC (Supplementary Methods). For FVC, the platelet-derived growth factor signalling, and the chromatin-packaging and -remodelling pathways were significant (P=2 × 10−4, false discovery rate (FDR)<0.3% and P=1.82 × 10−4, FDR<4%, respectively) (Supplementary Table 9).
Discussion
In this study, we aimed to improve coverage of low-frequency variants and detect novel loci associated with lung function, by undertaking imputation of GWAS data in 17 studies and 38,199 individuals to the 1000 Genomes Project18 reference panel, and by following up the most significant signals in an additional 54,550 individuals. Overall, 16 new association signals attained a genome-wide significance threshold corrected for multiple testing (P<5 × 10−8) after meta-analysing stage 1 and stage 2, including 15 autosomal and one X chromosome signal. While two of the new findings relate to novel signals for FEV1/FVC in previously reported regions10,12, five new loci were identified for FEV1, six new loci for FEV1/FVC and three new loci for FVC. Including the 16 signals discovered in these analyses, the number of lung function signals discovered to date is 49 (refs 10, 11, 12, 13, 14), and they jointly explain a modest proportion of the additive polygenic variance (4.0% for FEV1, 5.4% for FEV1/FVC and 3.2% for FVC).
Some of the 49 distinct lung function signals10,11,12,13,14 seem to cluster close to each other. If we define regions as 500 kb either side of the sentinel variants, there are three regions that each include two distinct signals (in or near INTS12-GSTCD-NPNT, GPR126 and PTCH1 (refs 10, 12)), so that the 49 signals would map to 46 loci. If we use a wider definition of region (1,000 kb either side of the sentinel), there are four regions that each include two distinct signals (in or near INTS12-GSTCD-NPNT, GPR126, PTCH1 (refs 10, 12) and TRIP11-RIN3). In addition, the human leukocyte antigen region on chromosome 6 includes three distinct signals (in or near ZKSCAN3-NCR3-AGER10,12,13) within 3.8 Mb. Furthermore, we have shown evidence of an additional signal in the TGFB2 region, and the new lung function signal in LTBP4 lies 179 kb away from a known COPD signal32. These findings are consistent with reports from very large studies of height and lipids53,54, which report multiple signals in associated regions, and highlight the importance of taking into account LD between variants to improve our understanding of known regions. Multiple signals within known regions are likely to explain some of the hidden heritability of these traits.
To identify pathways relevant to lung function, we undertook additional analyses using MAGENTA, which have implicated pathways for platelet-derived growth factor signalling and chromatin-packaging and -remodelling. Independent analyses undertaken in a concurrent study by the UK BiLEVE consortium, which focused on the extremes of the lung function distribution55, highlight the histone subset of the chromatin-packaging and -remodelling pathway. The TGFβ signalling pathway has now been implicated by three independent loci: an FEV1/FVC signal explained by a missense variant in LTBP4, which encodes a protein that binds TGFβ; an FEV1 signal upstream of TBX3, which is involved in the TGFβ1 signalling pathway34; and a previously reported signal downstream of TGFB2 (ref. 17). In addition, a pathway involving fibulin-5 has been implicated by two of the novel loci (LTBP4 and TRIP11). The identification, through different approaches, of pathways which appear to be involved in determining lung function should help focus future functional studies.
Pathways affecting lung function also have the potential to affect COPD risk, since lung function measures are used to diagnose the disease. Currently, 13 signals (in or near TGFB2, TNS1, RARB, FAM13A, GSTCD, HHIP, HTR4, ADAM19, AGER, LOC153910, C10orf11, RIN3 and THSD4) out of the 49 lung function signals discovered to date10,11,12,13,14 have also shown association with some definition of COPD38,56,57,58,59,60. This illustrates that the study of lung function measures is a powerful approach to bring insights into the genetics of COPD.
In agreement with previous findings for other lung function loci12,13, none of the 16 new associations seem to be driven by either smoking behaviour or by a gene–smoking interaction. One variant showed association with smoking behaviour that met a Bonferroni correction for 16 tests in UK BiLEVE. This variant also had an effect in never smokers in stage 1, and the allele associated with increased lung function was also associated with increased risk of smoking, which does not suggest an association with lung function mediated by smoking behaviour. Variants in five out of the 16 loci associated with lung function in this study have also shown associations with height44,45,46. However, the variants associated with height were either independent of those associated with lung function, or if they were correlated, the alleles associated with increased height, were associated with decreased FEV1 or FVC. If the association with lung function was driven by an effect on height, we would expect consistent direction of effect between these two traits. Therefore, the associations identified for lung function in these regions are not likely to be driven by associations with height.
This study had a large follow-up stage, which included 54,550 individuals, of which 48,943 were contributed by the UK BiLEVE study. UK BiLEVE is a particularly powerful study since it has sampled UK Biobank individuals from the extremes of the lung function distribution, and it has spirometry performed in a uniform way across individuals. Had these data been available when we undertook the discovery stage of this study, their addition would have greatly improved the discovery power. Nevertheless, incorporating these data into the follow-up stage improved power to provide replication and deal with potential winners’ curse bias. Another strength of the current study design was the increased coverage of common and low-frequency variants obtained through the imputation to 1000 Genomes Project18 reference panel. This enabled us to detect two low-allele-frequency variants (with MAF of 1.5 and 2.4% and stage-1 effect sizes of 0.17 and 0.16 s.d. units, respectively) that have an effect on lung function. No associations with lower allele frequency variants have been detected in this study, despite having power >80% in discovery to detect associations (P<5 × 10−6) for variants with MAF of 0.5 and 1%, and effect sizes above 0.3 and 0.2 s.d. units, respectively. The poorer imputation quality for low-allele-frequency variants coupled with the strict criteria we used to select variants for follow-up (N effective ⩾70%) have probably affected our ability to detect rare variants. For instance, a variant representing an additional signal for FEV1/FVC in the GSTCD-INTS12-NPNT region, reported by the UK BiLEVE study, where it was directly genotyped55, would have been detected in this analysis had we used a more lenient threshold (N effective >60%). Imputation quality for rare variants will improve as larger imputation reference panels become available.
In summary, 16 new association signals for lung function have been identified in this study, including two signals explained by non-synonymous low-frequency variants. These findings highlight new loci not previously connected with lung function or COPD, and bring new insights into previously detected loci. This study also highlights the added value of imputing to new reference panels as they become available. Understanding the molecular pathways that connect the newly identified loci with lung function and COPD risk has the potential to point to new targets for therapeutic intervention.
Methods
Study design
The study consisted of two stages. Stage 1 was a meta-analysis of 17 GWAS in a total of 38,199 individuals of European ancestry. Supplementary Table 1 gives the details of these studies. Fifty-six variants selected according to the results in stage 1 were followed up in stage 2 in 54,550 European individuals.
Stage-1 samples
Stage 1 comprised 17 studies: B58C (T1DGC and WTCCC), BHS1 and -2, EPIC (obese cases and population-based studies), the EUROSPAN studies (CROATIA-Korcula, ORCADES, CROATIA-Split and CROATIA-Vis), GS:SFHS, Health 2000, KORA F4, KORA S3, LBC1936, NFBC1966, NSPHS, SAPALDIA, SHIP and YFS (see Supplementary Table 1a for the definitions of all abbreviations). All participants provided written informed consent and studies were approved by local Research Ethics Committees and/or Institutional Review Boards. Measurements of spirometry for each study are described in the Supplementary Note. The genotyping platforms and quality-control criteria implemented by each study are described in Supplementary Table 1b.
Imputation
Imputation to the all ancestries 1000 Genomes Project18 Phase-1 reference panel released in March 2012 was undertaken using MACH61 and minimac62 or IMPUTE2 (ref. 63) with pre-imputation filters and parameters as shown in Supplementary Table 1b. Specific software guidelines were used to impute the non-pseudoautosomal part of the X chromosome. The pseudoautosomal part of the X chromosome was not included in these analyses. Variants were excluded if the imputation information, assessed using r2.hat (MACH and minimac) or .info (IMPUTE2), was <0.3.
Data transformation and association testing in stage 1
Linear regression of age, age2, sex, height and principal components for population structure was undertaken on FEV1, FEV1/FVC and FVC separately for ever smokers and never smokers. The residuals were transformed to ranks and then transformed to normally distributed z-scores. These transformed residuals were then used as the phenotype for association testing under an additive genetic model, separately for ever smokers and never smokers. For X chromosome analyses, residuals for males and females were analysed separately and dosages for males were coded 0 for 0 copies of the coded allele and 2 for 1 copy of the coded allele. The software used was specified in Supplementary Table 1b. Studies with related individuals analysed ever smokers and never smokers together adjusting the regression for ever-smoking status and used appropriate tests for association in related individuals, as described in the Supplementary Note.
Meta-analysis of stage-1 data
Quality-control checks on the stage-1 data were undertaken using GWAtoolbox64 and R version 3.0.2 (see URLs). All meta-analysis steps were undertaken using inverse variance-weighted fixed-effects meta-analysis. Effect estimates were flipped across studies so that the coded allele was the reference allele in the 1000 Genomes Project18 reference panel. For each study with unrelated individuals, autosomal chromosomes results were meta-analysed between ever smokers and never smokers. After that, all study-specific standard errors were corrected using genomic control19. Study-specific genomic inflation factor estimates are shown in Supplementary Table 1a. Finally, effect-size estimates and s.e. were combined across studies, and genomic control19 was applied again at the meta-analysis level. For the X chromosome, studies of unrelated individuals meta-analysed smoking strata estimates within sex strata and then meta-analysed pooled sex strata estimates. After that, genomic control19 was applied to each study and results were meta-analysed across studies. Genomic control19 was applied again after the meta-analysis. To describe the effect of imperfect imputation on power, for each variant we report the effective sample size (N effective), which is the sum of the study-specific products of the sample size and the imputation quality metric. Meta-analysis statistics and figures were produced using R version 3.0.2 (see URLs).
Selection of variants for stage 2
Variants with N effective <70% were filtered out before selecting variants for follow-up (8,916,621 variants remained after filtering). Independent regions (±500 kb from the sentinel variant) were selected for FEV1, FEV1/FVC and FVC if the sentinel SNP or indel had P<5 × 10−6. If the same variant was selected for different traits, it was followed up for all the traits. If two different variants were selected for different traits within the same region, or if any of the regions selected had already been identified in previous GWAS10,11,12,13,14 but the sentinel variant was different from that previously reported, conditional analyses were undertaken to assess whether the signals within the same regions were distinct. If previously reported sentinel SNPs for a region were strongly correlated (r2>0.9), we only conditioned on the SNP that had shown the strongest association. If two variants were selected for different traits within the same new region, both variants were taken forward if their P-value conditioning on the other variant was <5 × 10−6; if not, only the variant with the most significant P value was taken forward. Variants within known regions were only taken forward if their P value conditioned on the previously reported variant was <5 × 10−6. Conditional analyses were undertaken using GCTA65, and B58C data were used to estimate LD. In total, 56 variants (49 SNPs and seven indels) were taken forward for follow-up, two of which were distinct signals within previously reported regions10,12,13. These variants are listed in Supplementary Table 2. Previously reported signals10,11,12,13,14 were not followed up.
Stage-2 samples
The 48 SNPs and seven indels on autosomal chromosomes were followed up in up to 54,550 individuals from four studies with in silico data: ECRHS, PIVUS, TwinsUK and UK BiLEVE (see Supplementary Table 1a for the definitions of all abbreviations). All participants provided written informed consent and studies were approved by local Research Ethics Committees and/or Institutional Review Boards. One SNP in the chromosome X was followed up in 52,359 individuals from PIVUS, TwinsUK and UK BiLEVE. Measurements of spirometry for each study are described in the Supplementary Note.
Meta-analysis of stage-2 data
All stage-2 studies undertook linear regression of age, age2, sex, height, ever-smoking status and principal components for population structure, if available, on FEV1, FEV1/FVC and FVC, then the residuals were transformed to ranks and to normally distributed Z-scores. These transformed residuals were then used as the phenotype for association testing under an additive genetic model. For the X chromosome analyses, allele dosages for hemizygous males were coded as 2. Effect sizes were flipped to be consistent with the stage-1 estimates, using the reference allele in 1000 Genomes Project18 as the coded allele. Genomic control19 was applied for studies that undertook the analysis genome-wide. Effect estimates and s.e. were combined across the stage-2 studies using an inverse variance-weighted meta-analysis.
Combination of stage 1 and 2 and multiple testing correction
A meta-analysis of stage-1 and stage-2 results was undertaken using inverse variance-weighted meta-analysis. We take into account the multiple tests undertaken by describing an association as genome-wide significant if it has P<5 × 10−8. In addition, we assessed whether any of the findings achieved independent replication in stage 2 using a threshold corrected for the number of variants followed up (0.05/56=8.93 × 10−4).
Functional characterization of novel loci
A series of analyses were undertaken to provide insights into the expression of genes within the 16 loci (defined as ±1 Mb either side of the sentinel variant) represented here. Blood21 and lung tissue20 eQTL analyses were undertaken for variants in these loci that were in LD (r2>0.3) with the sentinel variant in the region. We assessed whether variants within these loci that were strongly correlated with the sentinel variants (r2>0.8) were in DNase hypersensitivity sites as defined by ENCODE22 for cells potentially relevant to lung function. We also carried out conditional analyses, using GCTA65, of sentinel variants conditioning on functional variants within the loci to assess whether the association signals were explained by functional variants (P value of the sentinel variant conditioned on the functional variant, conditional P, >0.01). Functional variants were defined using SIFT66, PolyPhen-2 (ref. 67), CADD68 and GWAVA69 databases. Additional analyses were undertaken for a subset of priority genes within the 16 loci (description of the selection is given in the Supplementary Methods). These included RNA-seq analyses to confirm messenger RNA expression in a lung-relevant cell (bronchial epithelium) and detect novel splice isoforms; assessment of differential expression across pseudoglandular and canalicular stages of human fetal lung development using gestational age as a continuous variable in linear regression25, and assessment of differences in expression levels in bronchial brushings between COPD cases and smoking controls70. Details for all these analyses are provided in the Supplementary Methods.
Associations with other traits
The association of the 16 sentinel variants with the following traits was assessed: lung function in children undertaking the same analysis as for adults in the ALSPAC data set42; gene by smoking interaction by undertaking a Z-test comparing the effect of a given variant in ever smokers and in never smokers using stage-1 results; smoking behaviour by undertaking a logistic regression analysis with heavy- versus never-smoking status as an outcome in the UK BiLEVE data set. In addition, the GWAS catalog43 was queried for variants in 2-Mb regions centred on the sentinel variant for the 16 loci. Variants that were genome-wide significant (P<5 × 10−8) in the GWAS catalog43 and were in LD (r2>0.3) with the sentinel variants, or were in genes that contained at least one variant in LD (r2>0.3) with the sentinel variants were selected.
Pathway analyses
Stage-1 GWAS results were tested for enrichment of known biological pathways using MAGENTA v2 (ref. 52). Six databases of biological pathways, including Ingenuity Pathway (June 2008, number of pathways n=81), KEGG (2010, n=186), PANTHER Molecular Function (January 2010, n=216), PANTHER Biological Processes (January 2010, n=217), PANTHER Pathways (January 2010, n=94) and Gene Ontology (April 2010, n=1778), were tested. An FDR threshold of 5% was used and significance thresholds were Bonferroni corrected for each database. Genes within 500 kb either side from the sentinel variants were flagged in the analysis. Sensitivity analyses were run after removing genes in the human leukocyte antigen region on chromosome 6. More details on the method are provided in the Supplementary Methods.
Additional analyses
Heterogeneity tests were undertaken for the 16 sentinel variants in stage 1. We undertook stepwise conditional analyses as performed by GCTA65 in each locus to identify additional signals. Full methods and results are described in the Supplementary Notes.
Additional information
How to cite this article: Artigas, M. S. et al. Sixteen new lung function signals identified through 1000 Genomes Project reference panel imputation. Nat. Commun. 6:8658 doi: 10.1038/ncomms9658 (2015).
References
Hole, D. J. et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ 313, 711–715 discussion 715–716 (1996).
Young, R. P., Hopkins, R. & Eaton, T. E. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur. Respir. J. 30, 616–622 (2007).
Lopez, A. D. et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur. Respir. J. 27, 397–412 (2006).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Zappala, C. J. et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur. Respir. J. 35, 830–836 (2010).
Abbey, D. E. et al. Long-term particulate and other air pollutants and lung function in nonsmokers. Am. J. Respir. Crit. Care Med. 158, 289–298 (1998).
Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis Management and Prevention of COPD. http://www.goldcopd.org/ (2014).
Wilk, J. B. et al. Evidence for major genes influencing pulmonary function in the NHLBI family heart study. Genet. Epidemiol. 19, 81–94 (2000).
Palmer, L. J. et al. Familial aggregation and heritability of adult lung function: results from the Busselton Health Study. Eur. Respir. J. 17, 696–702 (2001).
Hancock, D. B. et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat. Genet. 42, 45–52 (2010).
Loth, D. W. et al. Genome-wide association analysis identifies six new loci associated with forced vital capacity. Nat. Genet. 46, 669–677 (2014).
Repapi, E. et al. Genome-wide association study identifies five loci associated with lung function. Nat. Genet. 42, 36–44 (2010).
Soler Artigas, M. et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat. Genet. 43, 1082–1090 (2011).
Wilk, J. B. et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 5, e1000429 (2009).
Maher, B. Personal genomes: the case of the missing heritability. Nature 456, 18–21 (2008).
Manolio, T. A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009).
Gibson, G. Rare and common variants: twenty arguments. Nat. Rev. Genet. 13, 135–145 (2011).
1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
Devlin, B. & Roeder, K. Genomic control for association studies. Biometrics 55, 997–1004 (1999).
Lamontagne, M. et al. Refining susceptibility loci of chronic obstructive pulmonary disease with lung eqtls. PLoS ONE 8, e70220 (2013).
Westra, H. J. et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 45, 1238–1243 (2013).
Rosenbloom, K. R. et al. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res. 41, D56–D63 (2013).
Uhlen, M. et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 28, 1248–1250 (2010).
GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Melen, E. et al. Expression analysis of asthma candidate genes during human and murine lung development. Respir. Res. 12, 86 (2011).
Waller-Evans, H. et al. The orphan adhesion-GPCR GPR126 is required for embryonic development in the mouse. PLoS ONE 5, e14047 (2010).
Moriguchi, T. et al. DREG, a developmentally regulated G protein-coupled receptor containing two conserved proteolytic cleavage sites. Genes Cells 9, 549–560 (2004).
Paavola, K. J., Sidik, H., Zuchero, J. B., Eckart, M. & Talbot, W. S. Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci. Signal. 7, ra76 (2014).
Obeidat, M. e et al. GSTCD and INTS12 regulation and expression in the human lung. PLoS ONE 8, e74630 (2013).
Dabovic, B. et al. Function of latent TGFbeta binding protein 4 and fibulin 5 in elastogenesis and lung development. J. Cell. Physiol. 230, 226–236 (2015).
Sterner-Kock, A. et al. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 16, 2264–2273 (2002).
Cho, M. H. et al. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum. Mol. Genet. 21, 947–957 (2012).
Thorgeirsson, T. E. et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 42, 448–453 (2010).
Li, J. et al. The anti-proliferative function of the TGF-beta1 signalling pathway involves the repression of the oncogenic TBX2 by its homologue TBX3. J. Biol. Chem. 289, 35633–35643 (2014).
Follit, J. A. et al. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 4, e1000315 (2008).
Kawaguchi, Y. et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat. Genet. 8, 221–228 (1994).
Brandsma, C. A. et al. A large lung gene expression study identifying fibulin-5 as a novel player in tissue repair in COPD. Thorax 70, 21–32 (2015).
Cho, M. H. et al. Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. Lancet. Respir. Med. 2, 214–225 (2014).
Groenman, F., Rutter, M., Caniggia, I., Tibboel, D. & Post, M. Hypoxia-inducible factors in the first trimester human lung. J. Histochem. Cytochem. 55, 355–363 (2007).
Ovrevik, J. et al. AhR and Arnt differentially regulate NF-kappaB signaling and chemokine responses in human bronchial epithelial cells. Cell Commun. Signal. 12, 48 (2014).
Petrache, I. et al. Ceramide synthases expression and role of ceramide synthase-2 in the lung: insight from human lung cells and mouse models. PLoS ONE 8, e62968 (2013).
Boyd, A. et al. Cohort Profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 42, 111–127 (2013).
Welter, D. et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Research 42, D1001–D1006 (2014).
Lango Allen, H. et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467, 832–838 (2010).
Lettre, G. et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat. Genet. 40, 584–591 (2008).
Berndt, S. I. et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 45, 501–512 (2013).
Albagha, O. M. et al. Genome-wide association identifies three new susceptibility loci for Paget’s disease of bone. Nat. Genet. 43, 685–689 (2011).
Kemp, J. P. et al. Phenotypic dissection of bone mineral density reveals skeletal site specificity and facilitates the identification of novel loci in the genetic regulation of bone mass attainment. PLoS Genet. 10, e1004423 (2014).
Pei, Y. F. et al. Meta-analysis of genome-wide association data identifies novel susceptibility loci for obesity. Hum. Mol. Genet. 23, 820–830 (2014).
Macgregor, S. et al. Genome-wide association study identifies a new melanoma susceptibility locus at 1q21.3. Nat. Genet. 43, 1114–1118 (2011).
Porcu, E. et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 9, e1003266 (2013).
Segrè, A. V. et al. Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS Genet. 6, e1001058 (2010).
Wood, A. R. et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat. Genet. 46, 1173–1186 (2014).
Tada, H. et al. Multiple associated variants increase the heritability explained for plasma lipids and coronary artery disease. circulation. Cardiovasc. Genet. 7, 583–587 (2014).
Wain, L. V. et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir. Med. doi: 10.1016/S2213-2600(15)00283-0 (2015).
Soler Artigas, M. et al. Effect of five genetic variants associated with lung function on the risk of chronic obstructive lung disease, and their joint effects on lung function. Am. J. Respir. Crit. Care Med. 184, 786–795 (2011).
Castaldi, P. J. et al. The association of genome-wide significant spirometric loci with chronic obstructive pulmonary disease susceptibility. Am. J. Respir. Cell Mol. Biol. 45, 1147–1153 (2011).
Pillai, S. G. et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 5, e1000421 (2009).
Cho, M. H. et al. Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat. Genet. 42, 200–202 (2010).
Wilk, J. B. et al. Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction. Am. J. Respir. Crit. Care Med. 186, 622–632 (2012).
Li, Y. & Abecasis, G. R. Mach 1.0: rapid haplotype construction and missing genotype inference. Am. J. Hum. Genet. S79, 2290 (2006).
Howie, B., Fuchsberger, C., Stephens, M., Marchini, J. & Abecasis, G. R. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat. Genet. 44, 955–959 (2012).
Howie, B. N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
Fuchsberger, C., Taliun, D., Pramstaller, P. P., Pattaro, C. & consortium, C. K GWAtoolbox: an R package for fast quality control and handling of genome-wide association studies meta-analysis data. Bioinformatics 28, 444–445 (2012).
Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet. 44, S361–S363 (2012).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Adzhubei, I. A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Kircher, M. et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 46, 310–315 (2014).
Ritchie, G. R., Dunham, I., Zeggini, E. & Flicek, P. Functional annotation of noncoding sequence variants. Nat. Methods 11, 294–296 (2014).
Steiling, K. et al. A dynamic bronchial airway gene expression signature of chronic obstructive pulmonary disease and lung function impairment. Am. . Respir. Crit. Care Med. 187, 933–942 (2013).
Acknowledgements
The research undertaken by M.D.T., M.S.A. and L.V.W. was partly funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. M.D.T. holds a Medical Research Council Senior Clinical Fellowship (G0902313). This research used the ALICE High Performance Computing Facility at the University of Leicester. The Universities of Leicester and Nottingham acknowledge receipt of a Collaborative Research and Development grant from the Healthcare and Bioscience iNet, a project funded by the East Midlands Development Agency, part-financed by the European Regional Development Fund and delivered by Medilink East Midlands. I.P.H. holds a Medical Research Council programme grant (G1000861). We acknowledge the use of phenotype and genotype data from the British 1958 Birth Cohort DNA collection, funded by the Medical Research Council grant G0000934 and the Wellcome Trust grant 068545/Z/02 (http://www.b58cgene.sgul.ac.uk/). Genotyping for the B58C-WTCCC subset was funded by the Wellcome Trust grant 076113/B/04/Z. The B58C-T1DGC genotyping utilized resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, National Human Genome Research Institute, National Institute of Child Health and Human Development and Juvenile Diabetes Research Foundation International and supported by U01 DK062418. B58C-T1DGC GWAS data were deposited by the Diabetes and Inflammation Laboratory, Cambridge Institute for Medical Research (CIMR), University of Cambridge, which is funded by Juvenile Diabetes Research Foundation International, the Wellcome Trust and the National Institute for Health Research Cambridge Biomedical Research Centre; the CIMR is in receipt of a Wellcome Trust Strategic Award (079895). The B58C-GABRIEL genotyping was supported by a contract from the European Commission Framework Programme 6 (018996) and grants from the French Ministry of Research. The Busselton Health Study (BHS) acknowledges the generous support for the 1994/5 follow-up study from Healthway, Western Australia and the numerous Busselton community volunteers who assisted with data collection and the study participants from the Shire of Busselton. The BHS is supported by The Great Wine Estates of the Margaret River region of Western Australia. GWAS genotyping was supported by a research collaboration with Pfizer. The CROATIA study was supported through grants from the Medical Research Council UK, the Ministry of Science, Education and Sport in the Republic of Croatia (number 216-1080315-0302), Croatian Science Foundation (grant number 8875) and the European Union framework program 6 EUROSPAN project (contract no. LSHG-CT-2006-018947). SNP genotyping for CROATIA-Vis was performed by the Wellcome Trust Clinical Research Facility (WTCRF) at the Western General Hospital, Edinburgh, UK. CROATIA-Korcula was genotyped by Helmholz Zentrum München, GmbH, Neuherberg, Germany and CROATIA-Split by AROS Applied Biotechnology, Aarhus, Denmark. We would like to acknowledge the invaluable contributions of the recruitment teams in Croatia (including those from the Institute of Anthropological Research in Zagreb and the Croatian Centre for Global Health at the University of Split), the administrative teams in Croatia and Edinburgh and the people of Korcula, Vis and Split. The EPIC Norfolk Study is funded by program grants from the Medical Research Council UK and Cancer Research UK, and by additional support from the European Union, Stroke Association, British Heart Foundation, Department of Health, Food Standards Agency and the Wellcome Trust. GS:SFHS is funded by the Scottish Executive Health Department, Chief Scientist Office, grant number CZD/16/6. Exome array genotyping for GS:SFHS was funded by the Medical Research Council UK and performed at the Wellcome Trust Clinical Research Facility Genetics Core at Western General Hospital, Edinburgh, UK. We acknowledge the invaluable contributions of the families who took part in the Generation Scotland: Scottish Family Health Study, the general practitioners and Scottish School of Primary Care for their help in recruiting them and the whole Generation Scotland team, which includes academic researchers, IT staff, laboratory technicians, statisticians and research managers. This study was financially supported by the Medical Research Fund of the Tampere University Hospital. S.R. was supported by the Academy of Finland (251217 and 255847), Center of Excellence in Complex Disease Genetics, EU FP7 projects ENGAGE (201413) and BioSHaRE (261433), the Finnish Foundation for Cardiovascular Research, Biocentrum Helsinki and the Sigrid Juselius Foundation. The KORA authors acknowledge all members of field staffs who were involved in the planning and conduction of the KORA Augsburg studies, as well as all KORA study participants. The KORA research platform (KORA, Cooperative Health Research in the Region of Augsburg) was initiated and financed by the Helmholtz Zentrum München—German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research and by the State of Bavaria. The KORA-Age project was financed by the German Federal Ministry of Education and Research (BMBF FKZ 01ET0713 and 01ET1003A) as part of the ‘Health in old age’ program. Furthermore, KORA research was supported within the Munich Center of Health Sciences (MC Health), Ludwig-Maximilians-Universität, as part of LMUinnovativ. Further support was provided by the Competence Network ASCONET, subnetwork COSYCONET (FKZ 01GI0882). We thank the cohort participants who contributed to this study. Genotyping was supported by the UK’s Biotechnology and Biological Sciences Research Council (BBSRC) (ref. BB/F019394/1). Phenotype collection was supported by Research Into Ageing (continues as part of Age UK’s The Disconnected Mind project). The work was undertaken by The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the BBSRC and Medical Research Council (MRC) is gratefully acknowledged. We thank the late Professor Paula Rantakallio (launch of NFBC1966), and Ms Outi Tornwall and Ms MinttuJussila (DNA biobanking). NFBC1966 received financial support from the Academy of Finland (project grants 104781, 120315, 129269, 1114194 and 24300796), University Hospital Oulu, Biocenter, University of Oulu, Finland (75617), NHLBI grant 5R01HL087679-02 through the STAMPEED program (1RL1MH083268-01), NIH/NIMH (5R01MH63706:02), EU FP7 (HEALTH-F4-2007-201413), EU FP8 (277849) and Medical Research Council, UK (G0500539, G1002319 and G0600705). U.G. acknowledges Swedish Medical Research Council (K2007-66X-20270-01-3, 2012-2884), Foundation for Strategic Research (SSF) and European Commission FP6 STRP (LSHG-CT-2006-01947). Å.J. acknowledges Swedish Society for Medical Research. The ORCADES study was funded by the Chief Scientist Office of the Scottish Government, the Royal Society and the MRC Human Genetics Unit. DNA extraction was performed at the Wellcome Trust Clinical Research Facility in Edinburgh. Genotyping was funded by the European Union Framework Programme 6 EUROSPAN project. Study directorate: NM Probst-Hensch (PI; e/g); T. Rochat (p), C. Schindler (s), N. Künzli (e/exp), J.M. Gaspoz (c) Scientific team: J.C. Barthélémy (c), W. Berger (g), R. Bettschart (p), A. Bircher (a), C. Brombach (n), P.O. Bridevaux (p), L. Burdet (p), Felber Dietrich D. (e), M. Frey (p), U. Frey (pd), M.W. Gerbase (p), D. Gold (e), E. de Groot (c), W. Karrer (p), F. Kronenberg (g), B. Martin (pa), A. Mehta (e), D. Miedinger (o), M. Pons (p), F. Roche (c), T. Rothe (p), P. Schmid-Grendelmeyer (a), D. Stolz (p), A. Schmidt-Trucksäss (pa), J. Schwartz (e), A. Turk (p), A. von Eckardstein (cc) and E. Zemp Stutz (e). Scientific team at coordinating centers: M. Adam (e), I. Aguilera (exp), S. Brunner (s), D. Carballo (c), S. Caviezel (pa), I. Curjuric (e), A. Di Pascale (s), J. Dratva (e), R. Ducret (s), E. Dupuis Lozeron (s), M. Eeftens (exp), I. Eze (e), E. Fischer (g), M. Foraster (e), M. Germond (s), L. Grize (s), S. Hansen (e), A. Hensel (s), M. Imboden (g), A. Ineichen (exp), A. Jeong (g), D. Keidel (s), A. Kumar (g), N. Maire (s), A. Mehta (e), R. Meier (exp), E. Schaffner (s), T. Schikowski (e) and M. Tsai (exp); (a) allergology, (c) cardiology, (cc) clinical chemistry, (e) epidemiology, (exp) exposure, (g) genetic and molecular biology, (m) meteorology, (n) nutrition, (o) occupational health, (p) pneumology, (pa) physical activity, (pd) pediatrics and (s) statistic. The study could not have been done without the help of the study participants, technical and administrative support and the medical teams and field workers at the local study sites. Local field workers: Aarau: S. Brun, G. Giger, M. Sperisen and M. Stahel; Basel: C. Bürli, C. Dahler, N. Oertli, I. Harreh, F. Karrer, G. Novicic and N. Wyttenbacher; Davos: A. Saner, P. Senn and R. Winzeler; Geneva: F. Bonfils, B. Blicharz, C. Landolt and J. Rochat; Lugano: S. Boccia, E. Gehrig, M.T. Mandia, G. Solari and B. Viscardi; Montana: A.P. Bieri, C. Darioly and M. Maire; Payerne: F. Ding and P. Danieli A. Vonnez; Wald: D. Bodmer, E. Hochstrasser, R. Kunz, C. Meier, J. Rakic, U. Schafroth and A. Walder. Administrative staff: N. Bauer Ott, C. Gabriel, R. Gutknecht. Funding: The Swiss National Science Foundation (grants nos 33CS30-148470/1, 33CSCO-134276/1, 33CSCO-108796, 3247BO-104283, 3247BO-104288, 3247BO-104284, 3247-065896, 3100-059302, 3200-052720, 3200-042532, 4026-028099, PMPDP3_129021/1 and PMPDP3_141671/1), the Federal Office for the Environment, the Federal Office of Public Health, the Federal Office of Roads and Transport, the Canton’s Government of Aargau, Basel-Stadt, Basel-Land, Geneva, Luzern, Ticino, Valais and Zürich, the Swiss Lung League, the Canton’s Lung League of Basel-Stadt/ Basel Landschaft, Geneva, Ticino, Valais, Graubünden and Zurich, Stiftung ehemals Bündner Heilstätten, SUVA, Freiwillige Akademische Gesellschaft, UBS Wealth Foundation, Talecris Biotherapeutics GmbH, Abbott Diagnostics, European Commission 018996 (GABRIEL), and Wellcome Trust WT 084703MA. SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research, the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania, and the network ‘Greifswald Approach to Individualized Medicine (GANI_MED)’ funded by the Federal Ministry of Education and Research, and the German Asthma and COPD Network (COSYCONET) (grant nos 01ZZ9603, 01ZZ0103, 01ZZ0403, 03IS2061A and BMBF 01GI0883). Genome-wide data have been supported by the Federal Ministry of Education and Research and a joint grant from Siemens Healthcare, Erlangen, Germany and the Federal State of Mecklenburg-West Pomerania (grant no. 03ZIK012). The University of Greifswald is a member of the ‘Center of Knowledge Interchange’ program of the Siemens AG and the Caché Campus program of the InterSystems GmbH. We acknowledge the Academy of Finland (126925, 121584 and 124282), Academy of Finland (Eye) (134309), Academy of Finland (Salve) (129378), Academy of Finland (Gendi) (117787), Academy of Finland (Skidi) (41071), Social Insurance Institution of Finland,Tampere University Hospital Medical Funds (X51001 for T.L.), Kuopio University Hospital Medical Funds, Turku University Hospital Medical Funds, Juho Vainio Foundation, Paavo Nurmi Foundation, Finnish Foundation of Cardiovascular Research (T.L.), Finnish Cultural Foundation, Tuberculosis Foundation (T.L.), Emil Aaltonen Foundation (T.L.) and Yrjö Jahnsson Foundation (T.L.). We are extremely grateful to all the families who took part in the study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and the Wellcome Trust (grant ref: 092731) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and D.M.E. will serve as guarantor for the contents of this paper. This work was funded by a Medical Research Council (MRC) strategic award to M.D.T., I.P.H., D.P.S. and L.V.W. (MC_PC_12010). This research has been conducted using the UK Biobank Resource. M.D.T. has been supported by MRC fellowships G0501942 and G0902313. I.P.H. is supported by an MRC programme grant (G1000861). J.M. is funded by an ERC Consolidator Grant (617306). This article presents independent research funded partially by the NIHR. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. We would like to acknowledge all members of the UK Biobank Array Design Group. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Peter Donnelly (chair) (University of Oxford), Jeff Barrett (Wellcome Trust Sanger Institute), Jose Bras (University College London), Adam Butterworth (University of Cambridge), Richard Durbin (Wellcome Trust Sanger Institute), Paul Elliott (Imperial College London), Ian Hall (University of Nottingham), John Hardy (University College London), Mark McCarthy (University of Oxford), Gil McVean (University of Oxford), Tim Peakman (UK Biobank), Nazneen Rahman (The Institute of Cancer Research), Nilesh Samani (University of Leicester), Martin Tobin (University of Leicester), Hugh Watkins (University of Oxford). We acknowledge EU funding (GABRIEL Grant Number: 018996, ECRHS II Coordination Number: QLK4-CT-1999-01237). A.P.M. acknowledges the Wellcome Trust (WT098017,WT064890 and WT090532). The PIVUS study acknowledges The Swedish Foundation for Strategic Research (ICA08-0047), The Swedish Research Council (2012-1397), The Swedish Heart-Lung Foundation (20120197), The Swedish Society of Medicine and Uppsala University. The computations were performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project p2013056. A.P.M. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science (grant number WT098017). This cohort received funding from the Wellcome Trust; the European Community’s Seventh Framework Programme (FP7/2007-13); US National Institutes of Health/National Eye Institute (1RO1EY018246); NIH Center for Inherited Disease Research; the NIHR- funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London. We thank the research staff at the Respiratory Health Network Tissue Bank of the FRQS for collecting lung specimens for the lung eQTL study at the Laval University. The Lung Tissue eQTL study was funded by Merck Research Laboratories. M.O. is a Postdoctoral Fellow of the Michael Smith Foundation for Health Research and the Canadian Institute for Health Research Integrated and Mentored Pulmonary and Cardiovascular Training program (IMPACT). Y.B. is the recipient of a Junior 2 Research Scholar award from the Fonds de recherche Québec—Santé (FRQS).
Author information
Authors and Affiliations
Consortia
Contributions
I.P.H., S.M., N.S., M.S.A., D.P.S., M.D.T. and L.V.W. contributed to analysis. I.P.H, A.K.K., E.M., S.M., I.N., I.S., M.S.A., M.D.T. and L.V.W. carried out bioinformatics and functional assessment. I.P.H., S.M., M.S.A., D.P.S., M.D.T. and L.V.W. wrote the manuscript. Project conception, design and management: Stage 1—B58C: D.P.S.; CROATIA-Korcula: C.H.; CROATIA-Split: I.K., O.P., V.V. and T.Z.; CROATIA-Vis: I.R. and A.F.W.; EPIC: J.H.Z., R.A.S. and N.J.W.; GS:SFHS: L.H., S.P. and G.S.; H2000: M. Heliövaara and M.K.; KORA F4: J. Heinrich; KORA S3: C.G., S.K. and H.S.; LBC1936: I.J.D. and J.M.S.; NFBC1966: M.-R.J.; NSPHS: U.G.; ORCADES: H.C., S.H.W. and J.F.W.; SAPALDIA: N.M.P.-H.; SHIP: S.G., B.K. and H.V.; YFS: T.L. and O.T.R. Stage 2—ECRHS: J. Heinrich and D.L.J.; PIVUS: L.L.; TwinsUK: T.D.S. Phenotype collection and data management: Stage 1—B58C: W.L.M. and D.P.S.; BHS1&2: J.B., J. Hui, A.L.J. and A.W.M.; CROATIA-Korcula: C.H., J.E.H. and P.N.; CROATIA-Split: I.K., O.P., V.V. and T.Z.; CROATIA-Vis: J.M.; EPIC: J.H.Z.; GS:SFHS: L.H., S.P., G.S. and H.T.; H2000: M. Heliövaara, M.K., S.R. and I. Surakka; KORA F4: H.G. and J.S.R.; KORA S3: C.G., S.K., R.R. and H.S.; LBC1936: I.J.D., S.E.H., L.M.L. and J.M.S.; NFBC1966: A.C.A., A.-L.H. and M.-R.J.; NSPHS: S.E. and A.J.; ORCADES: H.C., S.H.W. and J.F.W.; SAPALDIA: M.I., A.K. and N.M.P.-H.; SHIP: S.G., B.S., A.T. and H.V.; YFS: N.H.-K., T.L. and O.T.R. Stage 2—ALSPAC: R.G., J. Henderson and J.P.K.; ECRHS: J.R.G., J. Heinrich and D.L.J.; PIVUS: E.I., L.L., A.M. and A.P.M.; TwinsUK: C.J.H., P.G.H., T.D.S. and A.V.; Lung eQTL study: Y.B., C.-A.B., D.C.N. and D.D.S. Data analysis: Stage 1—B58C: D.P.S.; CROATIA-Korcula: J.E.H. and P.N.; CROATIA-Vis: J.M.; EPIC: J.H.Z.; H2000: M.K., S.R. and I. Surakka; KORA F4: E.A. and J.S.R.; KORA S3: R.R.; LBC1936: L.M.L.; NFBC1966: A.C.A., M. Horikoshi and M.-R.J.; NSPHS: S.E.; ORCADES: P.K.J.; SAPALDIA: M.I., A.K. and N.M.P.-H.; SHIP: A.T.; YFS: L.-P.L. Stage 2—ALSPAC: D.M.E.; ECRHS: C.F.; PIVUS: A.M. and A.P.M.; TwinsUK: P.G.H; Lung eQTL study: M.O.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A full list of consortium members appears at the end of the paper.
Supplementary information
Supplementary Information
Supplementary Figures 1-5, Supplementary Tables 1-9, Supplementary Notes 1-4, Supplementary Methods and Supplementary References (PDF 5804 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Artigas, M., Wain, L., Miller, S. et al. Sixteen new lung function signals identified through 1000 Genomes Project reference panel imputation. Nat Commun 6, 8658 (2015). https://doi.org/10.1038/ncomms9658
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms9658
This article is cited by
-
Human genetic associations of the airway microbiome in chronic obstructive pulmonary disease
Respiratory Research (2024)
-
A mutation in CCDC91, Homo sapiens coiled-coil domain containing 91 protein, cause autosomal-dominant acrokeratoelastoidosis
European Journal of Human Genetics (2024)
-
Genomic prediction based on selective linkage disequilibrium pruning of low-coverage whole-genome sequence variants in a pure Duroc population
Genetics Selection Evolution (2023)
-
Genetic association and causal inference between lung function and venous thromboembolism
Respiratory Research (2023)
-
X chromosome associations with chronic obstructive pulmonary disease and related phenotypes: an X chromosome-wide association study
Respiratory Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.