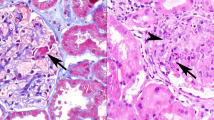
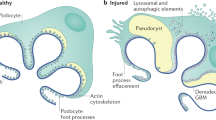
Abstract
Accumulating evidence indicates that recurrent glomerulonephritis is the third most important cause of renal allograft loss at 10 years after transplantation. The proteinuria and elevated serum creatinine levels that result from recurrent glomerulonephritis are associated with cardiovascular morbidity and mortality. The exact prevalence of either recurrent or de novo post-transplantation glomerulonephritis is unknown because a considerable number of patients never undergo allograft biopsy, meaning that glomerulonephritis remains undiagnosed and a diagnosis of 'chronic rejection/chronic allograft nephropathy' is sometimes presumed. The lack of consensus regarding evaluation of kidney transplant recipients who exhibit slow deterioration of graft function is a major reason for underdiagnosis. All forms of glomerular disease can recur after transplantation, but the likelihood of recurrence differs according to type. Focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, IgA nephropathy and idiopathic diarrhea-negative hemolytic uremic syndrome often recur. Membranous nephropathy, focal segmental glomerulosclerosis, anti-glomerular basement membrane nephritis associated with Alport syndrome, and drug-induced thrombotic microangiopathy are the most common forms of de novo glomerulonephritis. This Review discusses the prevalence, risk factors, pathogenesis, clinicopathological features, and effects on graft outcome of recurrent and de novo glomerulonephritis in renal allografts. Treatment options are briefly outlined.
Key Points
-
Approximately 40% of kidney transplant recipients develop clinically relevant proteinuria; the most common cause is 'chronic rejection/chronic allograft nephropathy', with post-transplantation glomerulonephritis and calcineurin-inhibitor toxicity being the second most common causes
-
Glomerulonephritis is considered recurrent when the form that affected the native kidney recurs in the transplanted kidney; de novo (new-onset) glomerulonephritis is that which occurs in a transplant recipient whose original kidney disease was either not glomerular or was of a different pathological type
-
Recurrent and de novo post-transplantation glomerulonephritis have the same pathological features as disease that occurs in the native kidney but frequently coexist with glomerular manifestations of acute or chronic rejection
-
The prevalence of post-transplantation glomerulonephritis is not precisely known, mainly because of incomplete examination of graft biopsy samples and the lack of consensus regarding investigation of late allograft dysfunction
-
Focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, IgA nephropathy and idiopathic diarrhea-negative hemolytic uremic syndrome recur frequently after transplantation; membranous nephropathy, focal segmental glomerulosclerosis, anti-glomerular basement membrane nephritis in patients with Alport syndrome, and drug-induced thrombotic microangiopathy are the most common de novo diseases
-
Differential diagnoses and risk factors for recurrence of glomerulonephritis after transplantation need to be better defined in order to enable prompt diagnosis and treatment; multicenter studies on the optimum treatment strategies are also needed
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yakupoglu U et al. (2004) Post-transplant nephrotic syndrome: a comprehensive clinicopathologic study. Kidney Int 65: 2360–2370
Chung J et al. (2000) Glomerulonephritis is the major cause of proteinuria in renal transplant recipients: histopathologic findings of renal allografts with proteinuria. Clin Transplant 14: 499–504
Suzuki H et al. (2003) Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 63: 2286–2294
Moriyama T et al. (2005) Latent IgA deposition from donor kidney is the major risk factor for recurrent IgA nephropathy in renal transplantation. Clin Transplant 19 (Suppl 14): S41–S48
Schwartzman MS et al. (2005) Transplantation and 6-month follow-up of renal transplantation from a donor with systemic lupus erythematosus and lupus nephritis. Am J Transplant 5: 1772–1776
Briggs JD and Jones E (1999) Recurrence of glomerulonephritis following renal transplantation. Nephrol Dial Transplant 14: 564–565
Chadban S (2001) Glomerulonephritis recurrence in the renal graft. J Am Soc Nephrol 12: 394–402
Hariharan S et al. (1998) Recurrent and de novo renal diseases after renal transplantation: a report from the Renal Allograft Disease Registry. Am J Kidney Dis 31: 928–931
Hariharan S et al. (1999) Recurrent and de novo glomerular disease after renal transplantation: a report from Renal Allograft Disease Registry (RADR). Transplantation 68: 635–641
Briganti EM et al. (2002) Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med 347: 103–109
Nankivell BJ et al. (2004) Evolution and pathophysiology of renal-transplant glomerulosclerosis. Transplantation 78: 461–468
Ibrahim H et al. (2006) Graft loss from recurrent glomerulonephritis is not increased with a rapid steroid discontinuation protocol. Transplantation 81: 214–219
Morozumi K et al. (2004) Cyclosporine nephrotoxicity: how does it affect renal allograft function and transplant morphology? Transplant Proc 36 (Suppl 2): 251S–256S
Cosio FG et al. (1999) Focal segmental glomerulosclerosis in renal allografts with chronic nephropathy: implications for graft survival. Am J Kidney Dis 34: 731–738
Zimmerman S (1984) Increased urinary protein excretion in the rat produced by serum from a patient with recurrent focal glomerular sclerosis after renal transplantation. Clin Nephrol 22: 32–38
Sharma M et al. (2002) Proteinuria after injection of human focal segmental glomerulosclerosis factor. Transplantation 73: 366–372
Musante L et al. (2002) Characterization of plasma factors that alter the permeability to albumin within isolated glomeruli. Proteomics 2: 197–205
Coward RJ et al. (2005) Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, podocin, and CD2 associated protein in cultured human podocytes. J Am Soc Nephrol 16: 629–637
Caridi G et al. (2003) Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol 14: 1278–1286
Caridi G et al. (2003) Podocin mutations in sporadic focal-segmental glomerulosclerosis occurring in adulthood. Kidney Int 64: 365
Ruf R et al. (2004) Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol 15: 722–732
Weber S et al. (2004) NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int 66: 571–579
Carraro M et al. (2002) Serum glomerular permeability activity in patients with podocin mutations (NPHS2) and steroid-resistant nephrotic syndrome. J Am Soc Nephrol 13: 1946–1952
Newstead CG (2003) Recurrent disease in renal transplants. Nephrol Dial Transplant 18 (Suppl 6): Svi68–Svi74
Swaminathan S et al. (2006) Collapsing and non-collapsing focal segmental glomerulosclerosis in kidney transplants. Nephrol Dial Transplant 21: 2607–2614
Adrogue HE et al. (2007) Coincident activation of Th2 T cells with onset of the disease and differential expression of GRO-gamma in peripheral blood leukocytes in minimal change disease. Am J Nephrol 27: 253–261
Zafarmand A et al. (2002) De novo minimal change disease associated with reversible post-transplant nephrotic syndrome: a report of five cases and review of literature. Clin Transplant 16: 350–361
Ronco P and Debiec H (2005) Molecular pathomechanisms of membranous nephropathy: from Heymann nephritis to alloimmunization. J Am Soc Nephrol 16: 1205–1213
Cosyns JP et al. (1998) Recurrence of membranous nephropathy after renal transplantation: probability, outcome and risk factors. Clin Nephrol 50: 144–153
Requiao-Moura R et al. (2007) Prognostic factors associated with poor graft outcomes in renal recipients with post-transplant glomerulonephritis. Clin Transplant 21: 363–370
Schwarz A et al. (1994) Impact of de novo membranous glomerulonephritis on the clinical course after kidney transplantation. Transplantation 58: 650–654
Poduval RD et al. (2003) Treatment of de novo and recurrent membranous nephropathy in renal transplant recipients. Semin Nephrol 23: 392–399
Joosten SA et al. (2005) Antibody response against the glomerular basement membrane protein agrin in patients with transplant glomerulopathy. Am J Transplant 5: 383–393
Berger SP and Daha MR (2007) Complement in glomerular injury. Semin Immunopathol 29: 375–384
Braun MC et al. (2005) Recurrence of membranoproliferative glomerulonephritis type II in renal allografts: the North American Pediatric Renal Transplant Cooperative Study Experience. J. Am Soc Nephrol 16: 2225–2233
Little MA et al. (2006) Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int 69: 504–511
Levy J et al. (2001) Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med 134: 1033–1042
Khandelwal M et al. (2004) Recurrence of anti-GBM disease 8 years after renal transplantation. Nephrol Dial Transplant 19: 491–494
Borza DB et al. (2005) Recurrent Goodpasture's disease secondary to a monoclonal IgA1-kappa antibody autoreactive with the alpha1/alpha2 chains of type IV collagen. Am J Kidney Dis 45: 397–406
Nachman PH et al. (1999) Recurrent ANCA-associated small vessel vasculitis after transplantation: a pooled analysis. Kidney Int 56: 1544–1550
Elmedhem A et al. (2003) Relapse rate and outcome of ANCA-associated small vessel vasculitis after transplantation. Nephrol Dial Transplant 18: 1001–1004
Choy BY et al. (2006) Recurrent glomerulonephritis after kidney transplantation. Am J Transplant 6: 2535–2542
Gera M et al. (2007) Recurrence of ANCA-associated vasculitis following renal transplantation in the modern era of immunosupression. Kidney Int 71: 1296–1301
Barratt J et al. (2007) Immunopathogenesis of IgAN. Semin Immunopathol 29: 427–443
Gough J et al. (2005) Recurrent and de novo glomerular immune-complex deposits in renal transplant biopsies. Arch Pathol Lab Med 129: 231–233
Bumgardner GL et al. (1998) Single-center long-term results of renal transplantation for IgA nephropathy. Transplantation 65: 1053–1060
Jeong HJ et al. (2004) IgA nephropathy in renal allografts—recurrence and graft dysfunction. Yonsei Med J 45: 1043–1048
Chacko B et al. (2007) Outcomes of renal transplantation in patients with immunoglobulin A nephropathy in India. J Postgrad Med 53: 92–95
Choy BY et al. (2003) Renal transplantation in patients with primary immunoglobulin A nephropathy. Nephrol Dial Transplant 18: 2399–2404
Andresdottir MB et al. (2001) Favorable outcome of renal transplantation in patients with IgA nephropathy. Clin Nephrol 56: 279–288
Wang AY et al. (2001) Recurrent IgA nephropathy in renal transplant allografts. Am J Kidney Dis 38: 588–596
McDonald S et al. (2006) Recurrence of IgA nephropathy among renal allograft recipients from living donors is greater among those with zero HLA mismatches. Transplantation 82: 759–762
Jeong HJ et al. (2004) Glomerular crescents are responsible for chronic graft dysfunction in post-transplant IgA nephropathy. Pathol Int 54: 837–842
Coppo R et al. (2007) Serological and genetic factors in early recurrence of IgA nephropathy after renal transplantation. Clin Transplant 21: 728–737
Kashtan CE (2006) Renal transplantation in patients with Alport syndrome. Pediatr Transplant 10: 651–657
Jais JP et al. (2003) X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol 14: 2603–2610
Artz MA et al. (2003) Renal transplantation in patients with hemolytic uremic syndrome: high rate of recurrence and increased incidence of acute rejections. Transplantation 76: 821–826
Caprioli J et al. (2006) Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment and outcome. Blood 108: 1267–1279
Ponticelli C and Banfi G (2006) Thrombotic microangiopathy after kidney transplantation. Transpl Int 19: 789–794
Sellier-Leclerc A et al. (2007) Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol 18: 2392–2400
Bresin E et al. (2006) Outcome of renal transplantation in patients with non-Shiga toxin-associated hemolytic uremic syndrome: prognostic significance of genetic background. Clin J Am Soc Nephrol 1: 88–99
Reynolds JC et al. (2003) Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis 42: 1058–1068
Karthikeyan V et al. (2003) Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. Am J Transplant 3: 1289–1294
Zarifian A et al. (1999) Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney Int 55: 2457–2466
Schwimmer J et al. (2003) De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis 41: 471–479
Opelz G et al. (2006) No improvement of patient or graft survival in transplant recipients treated with angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers: a collaborative transplant study report. J Am Soc Nephrol 17: 3257–3262
Weber S and Tönshoff B (2005) Recurrence of focal-segmental glomerulosclerosis in children after renal transplantation: clinical and genetic aspects. Transplantation 80 (Suppl): S128–S134
Vincenti F and Ghiggeri GM (2005) New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant 5: 1179–1185
Yabu JM et al. (2008) Rituximab failed to improve nephrotic syndrome in renal transplant recipients with recurrent focal segmental glomerulosclerosis. Am J Transplant 8: 222–227
Fine RN (2007) Recurrence of nephrotic syndrome/focal segmental glomerulosclerosis following renal transplantation in children. Pediatr Nephrol 22: 496–502
Ruggenenti P et al. (2006) Rituximab for idiopathic membranous nephropathy: who can benefit? Clin J Am Soc Nephrol 1: 738–748
Gallon L and Chhabra D (2006) Anti-CD20 monoclonal antibody (rituximab) for the treatment of recurrent idiopathic membranous nephropathy in a renal transplant patient. Am J Transplant 6: 3017–3021
Geetha D et al. (2007) Successful induction of remission with rituximab for relapse of ANCA-associated vasculitis post-kidney transplant: report of two cases. Am J Transplant 7: 2821–2825
Courtney AE et al. (2006) Does angiotensin blockade influence graft outcome in renal transplant recipients with IgA nephropathy? Nephrol Dial Transplant 21: 3550–3554
Meier-Kriesche HU et al. (2003) Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation 75: 1291–1295
Fernández-Fresnedo G et al. (2004) Proteinuria: a new marker of long-term graft and patient survival in kidney transplantation. Nephrol Dial Transplant 19 (Suppl 3): Siii47–Siii51
Magee C and Pascual M (2004) Update in renal transplantation. Arch Intern Med 164: 1373–1388
Matas AJ (2006) Recurrent disease after kidney transplantation—it is time to unite to address this problem! Am J Transplant 6: 2527–2528
Floege J (2003) Recurrent glomerulonephritis following renal transplantation: an update. Nephrol. Dial Transplant 18: 1260–1265
Noris M and Remuzzi G (2005) Hemolytic uremic syndrome. J Am Soc Nephrol 16: 1035–1050
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Ivanyi, B. A primer on recurrent and de novo glomerulonephritis in renal allografts. Nat Rev Nephrol 4, 446–457 (2008). https://doi.org/10.1038/ncpneph0854
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0854
This article is cited by
-
A rare cause of proteinuria after kidney transplantation: Answers
Pediatric Nephrology (2019)
-
Histopathological findings in transplanted kidneys
Renal Replacement Therapy (2017)
-
Recurrent proliferative glomerulonephritis with monoclonal immunoglobulin G deposits leads to rapid graft loss after kidney transplantation: a case report
CEN Case Reports (2014)
-
Impact of Posttransplantation Glomerulonephritis on Long‐term Outcome of Kidney Transplants: Single‐Center 20‐Year Experience
World Journal of Surgery (2012)
-
Nierentransplantatpathologie
Der Pathologe (2011)