Abstract
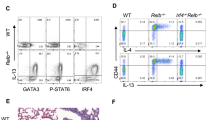
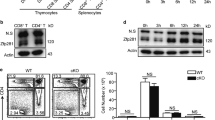
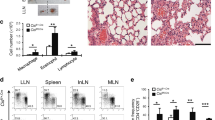
CD4+ helper T cells acquire effector phenotypes that promote specialized inflammatory responses. We show that the ETS-family transcription factor PU.1 was required for the development of an interleukin 9 (IL-9)-secreting subset of helper T cells. Decreasing PU.1 expression either by conditional deletion in mouse T cells or the use of small interfering RNA in human T cells impaired IL-9 production, whereas ectopic PU.1 expression promoted IL-9 production. Mice with PU.1-deficient T cells developed normal T helper type 2 (TH2) responses in vivo but showed attenuated allergic pulmonary inflammation that corresponded to lower expression of Il9 and chemokines in peripheral T cells and in lungs than that of wild-type mice. Together our data suggest a critical role for PU.1 in generating the IL-9-producing (TH9) phenotype and in the development of allergic inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dong, C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 8, 337–348 (2008).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Weaver, C.T., Harrington, L.E., Mangan, P.R., Gavrieli, M. & Murphy, K.M. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24, 677–688 (2006).
Ansel, K.M., Djuretic, I., Tanasa, B. & Rao, A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu. Rev. Immunol. 24, 607–656 (2006).
Lee, G.R., Kim, S.T., Spilianakis, C.G., Fields, P.E. & Flavell, R.A. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity 24, 369–379 (2006).
Murphy, K.M. & Reiner, S.L. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2, 933–944 (2002).
Chang, H.C. et al. PU.1 regulates TCR expression by modulating GATA-3 activity. J. Immunol. 183, 4887–4894 (2009).
Chang, H.C. et al. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity 22, 693–703 (2005).
Ahyi, A.N., Chang, H.C., Dent, A.L., Nutt, S.L. & Kaplan, M.H. IFN regulatory factor 4 regulates the expression of a subset of Th2 cytokines. J. Immunol. 183, 1598–1606 (2009).
Hauber, H.P., Bergeron, C. & Hamid, Q. IL-9 in allergic inflammation. Int. Arch. Allergy Immunol. 134, 79–87 (2004).
Forbes, E.E. et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J. Exp. Med. 205, 897–913 (2008).
Temann, U.A., Geba, G.P., Rankin, J.A. & Flavell, R.A. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J. Exp. Med. 188, 1307–1320 (1998).
Temann, U.A., Ray, P. & Flavell, R.A. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J. Clin. Invest. 109, 29–39 (2002).
Steenwinckel, V. et al. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J. Immunol. 182, 4737–4743 (2009).
Steenwinckel, V. et al. IL-13 mediates in vivo IL-9 activities on lung epithelial cells but not on hematopoietic cells. J. Immunol. 178, 3244–3251 (2007).
Temann, U.A., Laouar, Y., Eynon, E.E., Homer, R. & Flavell, R.A. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. Int. Immunol. 19, 1–10 (2007).
Townsend, J.M. et al. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13, 573–583 (2000).
Cheng, G. et al. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am. J. Respir. Crit. Care Med. 166, 409–416 (2002).
McMillan, S.J., Bishop, B., Townsend, M.J., McKenzie, A.N. & Lloyd, C.M. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J. Exp. Med. 195, 51–57 (2002).
Erpenbeck, V.J. et al. Segmental allergen challenge in patients with atopic asthma leads to increased IL-9 expression in bronchoalveolar lavage fluid lymphocytes. J. Allergy Clin. Immunol. 111, 1319–1327 (2003).
Shimbara, A. et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J. Allergy Clin. Immunol. 105, 108–115 (2000).
White, B., Leon, F., White, W. & Robbie, G. Two first-in-human, open-label, phase I dose-escalation safety trials of MEDI-528, a monoclonal antibody against interleukin-9, in healthy adult volunteers. Clin. Ther. 31, 728–740 (2009).
Faulkner, H., Renauld, J.C., Van Snick, J. & Grencis, R.K. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect. Immun. 66, 3832–3840 (1998).
Veldhoen, M. et al. Transforming growth factor-β 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat. Immunol. 9, 1341–1346 (2008).
Elyaman, W. et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. USA 106, 12885–12890 (2009).
Nowak, E.C. et al. IL-9 as a mediator of Th17-driven inflammatory disease. J. Exp. Med. 206, 1653–1660 (2009).
Schmitt, E. et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-β and IL-4, and is inhibited by IFN-γ. J. Immunol. 153, 3989–3996 (1994).
Lu, L.F. et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442, 997–1002 (2006).
Dardalhon, V. et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+IL-10+Foxp3− effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Tepper, R.S. et al. Expired nitric oxide and airway reactivity in infants at risk for asthma. J. Allergy Clin. Immunol. 122, 760–765 (2008).
Zhou, L., Chong, M.M. & Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Djuretic, I.M. et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat. Immunol. 8, 145–153 (2007).
Mullen, A.C. et al. Hlx is induced by and genetically interacts with T-bet to promote heritable TH1 gene induction. Nat. Immunol. 3, 652–658 (2002).
Thieu, V.T. et al. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper 1 cell-fate determination. Immunity 29, 679–690 (2008).
Brustle, A. et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 8, 958–966 (2007).
Schnyder-Candrian, S. et al. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 203, 2715–2725 (2006).
Gonzalo, J.A. et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 163, 403–411 (1999).
Kawasaki, S. et al. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J. Immunol. 166, 2055–2062 (2001).
Mikhak, Z. et al. Contribution of CCR4 and CCR8 to antigen-specific T(H)2 cell trafficking in allergic pulmonary inflammation. J. Allergy Clin. Immunol. 123, 67–73 (2009).
Wills-Karp, M. Interleukin-13 in asthma pathogenesis. Immunol. Rev. 202, 175–190 (2004).
Dakic, A. et al. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J. Exp. Med. 201, 1487–1502 (2005).
Stritesky, G.L., Yeh, N. & Kaplan, M.H. IL-23 mediates stability but not commitment to the Th17 lineage. J. Immunol. 181, 5948–5955 (2008).
van Rijt, L.S. et al. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J. Immunol. Methods 288, 111–121 (2004).
Acknowledgements
Supported by the National Institutes of Health (R01 AI57459 and U19 AI070448 to M.H.K., R01 CA118118 to M.J.R.; R01 HL080071 to R.S.T.; and T32 AI060519 to G.L.S.).
Author information
Authors and Affiliations
Contributions
M.H.K. designed and supervised the study and wrote the manuscript; H.-C.C., R.G., R.J. and L.H. did experiments in Figures 1,2,3 and Supplementary Figure 1; Q.Y., R.G. and G.L.S. did experiments in Figure 4 and Supplementary Figure 2; W.Y., M.J.R. and R.S.T. obtained human samples; W.Y. did all experiments in Figure 5; S.S., E.T.N., C.M. and A.-N.A. did experiments in Figures 6 and 7 and Supplementary Figure 3; N.B.P. provided bioinformatics analysis; and S.L.N. provided mice.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 102 kb)
Rights and permissions
About this article
Cite this article
Chang, HC., Sehra, S., Goswami, R. et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol 11, 527–534 (2010). https://doi.org/10.1038/ni.1867
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1867
This article is cited by
-
Haemonchus contortus HcL6 promoted the Th9 immune response in goat PBMCs by activating the STAT6/PU.1/NF-κB pathway
Veterinary Research (2023)
-
PPAR-γ regulates the effector function of human T helper 9 cells by promoting glycolysis
Nature Communications (2023)
-
IL-9 aggravates SARS-CoV-2 infection and exacerbates associated airway inflammation
Nature Communications (2023)
-
Role of LINC00240 on T-helper 9 differentiation in allergic rhinitis through influencing DNMT1-dependent methylation of PU.1
Immunologic Research (2023)
-
IL-9-Producing Th9 Cells Participate in the Occurrence and Development of Iodine-Induced Autoimmune Thyroiditis
Biological Trace Element Research (2023)