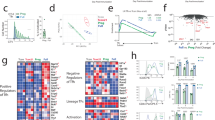
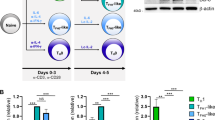
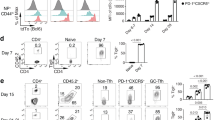
Abstract
Germinal centers require CD4+ follicular helper T cells (TFH cells), whose hallmark is expression of the transcriptional repressor Bcl-6, the chemokine receptor CXCR5 and interleukin 21 (IL-21). To track the development and fate of TFH cells, we generated an IL-21 reporter mouse by introducing sequence encoding green fluorescent protein (GFP) into the Il21 locus; these mice had expression of IL-21–GFP in CD4+CXCR5+PD-1+ TFH cells. IL-21–GFP+ TFH cells were multifunctional helper cells that coexpressed several cytokines, including interferon-γ (IFN-γ), IL-2 and IL-4. TFH cells proliferated and gave rise to transferrable memory cells with plasticity, which differentiated after recall into conventional effector helper T cells and TFH cells. Thus, we demonstrated that TFH cells were not terminally differentiated but instead retained the flexibility to be recruited into other helper T cell subsets and nonlymphoid tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zhu, J., Yamane, H. & Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Murphy, K.M. & Stockinger, B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat. Immunol. 11, 674–680 (2010).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Yu, D. & Vinuesa, C.G. The elusive identity of T follicular helper cells. Trends Immunol. 31, 377–383 (2010).
Nutt, S.L. & Tarlinton, D.M. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat. Immunol. 12, 472–477 (2011).
Ansel, K.M., McHeyzer-Williams, L.J., Ngo, V.N., McHeyzer-Williams, M.G. & Cyster, J.G. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 190, 1123–1134 (1999).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Campbell, D.J., Kim, C.H. & Butcher, E.C. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat. Immunol. 2, 876–881 (2001).
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
Nurieva, R.I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Coquet, J.M. et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol. 178, 2827–2834 (2007).
Zhou, L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Lee, S.K. et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J. Exp. Med. 208, 1377–1388 (2011).
Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Linterman, M.A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Akiba, H. et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 175, 2340–2348 (2005).
Johnston, R.J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Haynes, N.M. et al. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 179, 5099–5108 (2007).
Deenick, E.K. et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 33, 241–253 (2010).
Vogelzang, A. et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008).
Nurieva, R.I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Fazilleau, N. et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat. Immunol. 8, 753–761 (2007).
Dienz, O. et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 206, 69–78 (2009).
Suto, A. et al. Development and characterization of IL-21-producing CD4+ T cells. J. Exp. Med. 205, 1369–1379 (2008).
Kwon, H. et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity 31, 941–952 (2009).
Huber, M. et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc. Natl. Acad. Sci. USA 105, 20846–20851 (2008).
Yusuf, I. et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J. Immunol. 185, 190–202 (2010).
Zaretsky, A.G. et al. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med. 206, 991–999 (2009).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Oxenius, A., Bachmann, M.F., Zinkernagel, R.M. & Hengartner, H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28, 390–400 (1998).
Pepper, M., Pagan, A.J., Igyarto, B.Z., Taylor, J.J. & Jenkins, M.K. Opposing signals from the bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595 (2011).
Reinhardt, R.L., Liang, H.E. & Locksley, R.M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10, 385–393 (2009).
Kitano, M. et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity 34, 961–972 (2011).
Choi, Y.S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011).
Bauquet, A.T. et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 10, 167–175 (2009).
Campbell, D.J. & Koch, M.A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 11, 119–130 (2011).
Fazilleau, N., McHeyzer-Williams, L.J. & McHeyzer-Williams, M.G. Local development of effector and memory T helper cells. Curr. Opin. Immunol. 19, 259–267 (2007).
Baumjohann, D., Okada, T. & Ansel, K.M. Cutting edge: distinct waves of BCL6 expression during T follicular helper cell development. J. Immunol. 187, 2089–2092 (2011).
Neurath, M.F. et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J. Exp. Med. 195, 1129–1143 (2002).
Malissen, M. et al. Altered T cell development in mice with a targeted mutation of the CD3ɛ gene. EMBO J. 14, 4641–4653 (1995).
Flynn, K.J. et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8, 683–691 (1998).
Acknowledgements
We thank L. Corcoran (Walter and Eliza Hall Institute of Medical Research) for antibody to Bcl-6 and antibody to IRF4; and E. Cretney for preliminary experiments. Supported by the National Health and Medical Research Council of Australia (A.K., G.T.B., D.M.T. and S.L.N.), the German Academic Exchange Service (K.L.), the Sylvia and Charles Viertel Foundation (G.T.B.), the Howard Hughes Medical Institute (G.T.B.), Pfizer Australia Research (S.L.N.), the Australian Research Council (A.K. and S.L.N.), the Victorian State Government Operational Infrastructure Support and the National Health and Medical Research Council Independent Research Institutes Infrastructure Support Scheme of the Australian Government.
Author information
Authors and Affiliations
Contributions
K.L., A.K., Y.S., G.T.B., A.L., D.M.T. and S.L.N. designed and did experiments; and K.L., D.M.T. and S.L.N. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Tables 1–2 (PDF 845 kb)
Rights and permissions
About this article
Cite this article
Lüthje, K., Kallies, A., Shimohakamada, Y. et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol 13, 491–498 (2012). https://doi.org/10.1038/ni.2261
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2261
This article is cited by
-
CD4+ T cell memory
Nature Immunology (2023)
-
MiR-29a-3p negatively regulates circulating Tfh memory cells in patients with Graves’ disease by targeting ICOS
Immunologic Research (2023)
-
The phosphatase PTEN links platelets with immune regulatory functions of mouse T follicular helper cells
Nature Communications (2022)
-
Transcription tipping points for T follicular helper cell and T-helper 1 cell fate commitment
Cellular & Molecular Immunology (2021)
-
The concerted change in the distribution of cell cycle phases and zone composition in germinal centers is regulated by IL-21
Nature Communications (2021)