Abstract
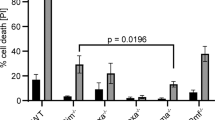
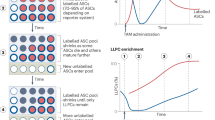
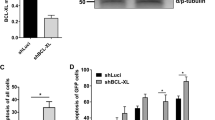
The long-term survival of plasma cells is entirely dependent on signals derived from their environment. These extrinsic factors presumably induce and sustain the expression of antiapoptotic proteins of the Bcl-2 family. It is uncertain whether there is specificity among Bcl-2 family members in the survival of plasma cells and whether their expression is linked to specific extrinsic factors. We found here that deletion of the gene encoding the antiapoptotic protein Mcl-1 in plasma cells resulted in rapid depletion of this population in vivo. Furthermore, we found that the receptor BCMA was needed to establish high expression of Mcl-1 in bone marrow but not spleen plasma cells and that establishing this survival pathway preceded the component of plasma cell differentiation that depends on the transcriptional repressor Blimp-1. Our results identify a critical role for Mcl-1 in the maintenance of plasma cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Change history
21 March 2013
In the version of this article initially published, the number for the National Health and Medical Research Council grant to I.V. is incorrect in the Acknowledgements section. The correct number is 1021374. The error has been corrected in the HTML and PDF versions of the article.
References
Shaffer, A.L. et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Shapiro-Shelef, M. et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19, 607–620 (2003).
Kallies, A. et al. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity 26, 555–566 (2007).
Kallies, A. et al. Plasma cell ontogeny defined by quantitative changes in Blimp-1 expression. J. Exp. Med. 200, 967–977 (2004).
Sze, D.M., Toellner, K.M., Garcia de Vinuesa, C., Taylor, D.R. & MacLennan, I.C. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J. Exp. Med. 192, 813–821 (2000).
Tarlinton, D. B-cell memory: are subsets necessary? Nat. Rev. Immunol. 6, 785–790 (2006).
Rajewsky, K. Clonal selection and learning in the antibody system. Nature 381, 751–758 (1996).
Kabashima, K. et al. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J. Exp. Med. 203, 2683–2690 (2006).
Tarlinton, D., Radbruch, A., Hiepe, F. & Dorner, T. Plasma cell differentiation and survival. Curr. Opin. Immunol. 20, 162–169 (2008).
Oracki, S.A., Walker, J.A., Hibbs, M.L., Corcoran, L.M. & Tarlinton, D.M. Plasma cell development and survival. Immunol. Rev. 237, 140–159 (2010).
Smith, K.G., Hewitson, T.D., Nossal, G.J. & Tarlinton, D.M. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 26, 444–448 (1996).
O'Connor, B.P. et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199, 91–98 (2004).
Benson, M.J. et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 180, 3655–3659 (2008).
Cassese, G. et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171, 1684–1690 (2003).
Rodriguez Gomez, M. et al. Basophils support the survival of plasma cells in mice. J. Immunol. 185, 7180–7185 (2010).
Rozanski, C.H. et al. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J. Exp. Med. 208, 1435–1446 (2011).
Tokoyoda, K., Egawa, T., Sugiyama, T., Choi, B.I. & Nagasawa, T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 20, 707–718 (2004).
Le Gouill, S., Podar, K., Harousseau, J.L. & Anderson, K.C. Mcl-1 regulation and its role in multiple myeloma. Cell Cycle 3, 1259–1262 (2004).
Potter, M. Neoplastic development in plasma cells. Immunol. Rev. 194, 177–195 (2003).
Belnoue, E. et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 111, 2755–2764 (2008).
Moreaux, J. et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 103, 3148–3157 (2004).
Carrington, E.M. et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc. Natl. Acad. Sci. USA 107, 10967–10971 (2010).
Mérino, D. et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 119, 5807–5816 (2012).
Vikstrom, I. et al. Mcl-1 is essential for germinal center formation and B cell memory. Science 330, 1095–1099 (2010).
Tarte, K. et al. The Bcl-2 family member Bfl-1/A1 is strongly repressed in normal and malignant plasma cells but is a potent anti-apoptotic factor for myeloma cells. Br. J. Haematol. 125, 373–382 (2004).
Tangye, S.G. Staying alive: regulation of plasma cell survival. Trends Immunol. 32, 595–602 (2011).
Jiang, C., Loo, W.M., Greenley, E.J., Tung, K.S. & Erickson, L.D. B cell maturation antigen deficiency exacerbates lymphoproliferation and autoimmunity in murine lupus. J. Immunol. 186, 6136–6147 (2011).
Good, K.L., Avery, D.T. & Tangye, S.G. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J. Immunol. 182, 890–901 (2009).
Glaser, S.P. et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 26, 120–125 (2012).
Nojima, T. et al. In vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat. Commun. 2, 465 (2011).
Vieira, P. & Rajewsky, K. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 18, 313–316 (1988).
Amanna, I.J., Carlson, N.E. & Slifka, M.K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357, 1903–1915 (2007).
Hammarlund, E. et al. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9, 1131–1137 (2003).
Crotty, S. et al. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171, 4969–4973 (2003).
Radbruch, A. et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6, 741–750 (2006).
Chu, V.T. et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12, 151–159 (2011).
Mohr, E. et al. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J. Immunol. 182, 2113–2123 (2009).
Huard, B. et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J. Clin. Invest. 118, 2887–2895 (2008).
Sciammas, R. et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 25, 225–236 (2006).
Shaffer, A.L. et al. IRF4 addiction in multiple myeloma. Nature 454, 226–231 (2008).
Manz, R.A., Hauser, A.E., Hiepe, F. & Radbruch, A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23, 367–386 (2005).
Novak, A.J. et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood 103, 689–694 (2004).
Tangye, S.G., Bryant, V.L., Cuss, A.K. & Good, K.L. BAFF, APRIL and human B cell disorders. Semin. Immunol. 18, 305–317 (2006).
Wuillème-Toumi, S. et al. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 19, 1248–1252 (2005).
van Vollenhoven, R.F., Kinnman, N., Vincent, E., Wax, S. & Bathon, J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 63, 1782–1792 (2011).
Genovese, M.C., Kinnman, N., de La Bourdonnaye, G., Pena Rossi, C. & Tak, P.P. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum. 63, 1793–1803 (2011).
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
Borriello, F. et al. B7–1 and B7–2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6, 303–313 (1997).
Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K.A. B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350, 423–426 (1991).
Janas, M.L., Hodgkin, P., Hibbs, M. & Tarlinton, D. Genetic evidence for Lyn as a negative regulator of IL-4 signaling. J. Immunol. 163, 4192–4198 (1999).
Acknowledgements
We thank the facilities of our respective institutes, particularly those responsible for animal husbandry and flow cytometry; and C. Wellard, S. Chevrier, D. Segal, D. Huang, S. Heinzel, J. Marchingo, S. Willis and P. Bouillet for assistance. Supported by the Australia National Health and Medical Research Council (1021374 to I.V.; 356202 to D.M.T. and S.L.N.; 461221 to A.S.; 637326 to S.P.G.; 516786 to K.F.; and the Independent Research Institute Infrastructure Support Scheme), the Multiple Myeloma Research Foundation USA (V.P.), the European Molecular Biology Organization (V.P.), the US National Institutes of Health (AI093722 to L.D.E.) and Victorian State Government Operational Infrastructure Support.
Author information
Authors and Affiliations
Contributions
V.P., I.V. and D.M.T. designed the research; V.P., I.V., J.W., S.P.G., M.L., C.M.C. and K.F. did experiments and contributed to interpretation and discussion; L.D.E., F.M., A.S. and S.L.N. contributed to the design of experiments, interpretation of results, and drafting the manuscript; V.P. and I.V. analyzed data and prepared figures; and V.P., I.V. and D.M.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 484 kb)
Rights and permissions
About this article
Cite this article
Peperzak, V., Vikström, I., Walker, J. et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol 14, 290–297 (2013). https://doi.org/10.1038/ni.2527
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2527
This article is cited by
-
1q21+ is associated with poor prognosis in newly diagnosed multiple myeloma patients with extramedullary disease: a retrospective study
Annals of Hematology (2024)
-
Differential expression pattern of Bcl-2 family members in B and T cells in systemic lupus erythematosus and rheumatoid arthritis
Arthritis Research & Therapy (2023)
-
BCL-2 protein family: attractive targets for cancer therapy
Apoptosis (2023)
-
Supplying the trip to antibody production—nutrients, signaling, and the programming of cellular metabolism in the mature B lineage
Cellular & Molecular Immunology (2022)
-
Differential expression of circulating miRNAs after alemtuzumab induction therapy in lung transplantation
Scientific Reports (2022)