Abstract
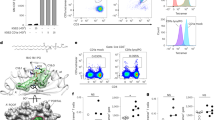
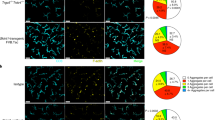
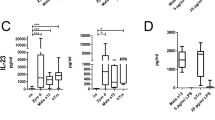
T cells autoreactive to the antigen-presenting molecule CD1a are common in human blood and skin, but the search for natural autoantigens has been confounded by background T cell responses to CD1 proteins and self lipids. After capturing CD1a-lipid complexes, we gently eluted ligands while preserving non–ligand-bound CD1a for testing lipids from tissues. CD1a released hundreds of ligands of two types. Inhibitory ligands were ubiquitous membrane lipids with polar head groups, whereas stimulatory compounds were apolar oils. We identified squalene and wax esters, which naturally accumulate in epidermis and sebum, as autoantigens presented by CD1a. The activation of T cells by skin oils suggested that headless mini-antigens nest within CD1a and displace non-antigenic resident lipids with large head groups. Oily autoantigens naturally coat the surface of the skin; thus, this points to a previously unknown mechanism of barrier immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Porcelli, S. et al. Recognition of cluster of differentiation 1 antigens by human CD4–CD8-cytolytic T lymphocytes. Nature 341, 447–450 (1989).
de Jong, A. et al. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat. Immunol. 11, 1102–1109 (2010).
de Lalla, C. et al. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur. J. Immunol. 41, 602–610 (2011).
Roura-Mir, C. et al. CD1a and CD1c activate intrathyroidal T cells during Graves' disease and Hashimoto's thyroiditis. J. Immunol. 174, 3773–3780 (2005).
Colonna, M. Skin function for human CD1a-reactive T cells. Nat. Immunol. 11, 1079–1080 (2010).
Shamshiev, A. et al. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29, 1667–1675 (1999).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Fox, L.M. et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 7, e1000228 (2009).
Brennan, P.J. et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 12, 1202–1211 (2011).
Van Rhijn, I. et al. CD1d-restricted T cell activation by nonlipidic small molecules. Proc. Natl. Acad. Sci. USA 101, 13578–13583 (2004).
Garboczi, D.N. et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 384, 134–141 (1996).
Garcia, K.C. et al. An αβ T cell receptor structure at 2.5 Å and its orientation in the TCR-MHC complex. Science 274, 209–219 (1996).
Borg, N.A. et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448, 44–49 (2007).
Altman, J.D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Kasmar, A.G. et al. CD1b tetramers bind αβ T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J. Exp. Med. 208, 1741–1747 (2011).
Porcelli, S.A. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 59, 1–98 (1995).
Zajonc, D.M. et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity 22, 209–219 (2005).
Moody, D.B. et al. T cell activation by lipopeptide antigens. Science 303, 527–531 (2004).
Ernst, W.A. et al. Molecular interaction of CD1b with lipoglycan antigens. Immunity 8, 331–340 (1998).
Deng, L. & Mariuzza, R.A. Recognition of self-peptide-MHC complexes by autoimmune T-cell receptors. Trends Biochem. Sci. 32, 500–508 (2007).
Cox, D. et al. Determination of cellular lipids bound to human CD1d molecules. PLoS ONE 4, e5325 (2009).
Yuan, W., Kang, S.J., Evans, J.E. & Cresswell, P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J. Immunol. 182, 4784–4791 (2009).
Haig, N.A. et al. Identification of self-lipids presented by CD1c and CD1d proteins. J. Biol. Chem. 286, 37692–37701 (2011).
Huang, S. et al. Discovery of deoxyceramides and diacylglycerols as CD1b scaffold lipids among diverse groove-blocking lipids of the human CD1 system. Proc. Natl. Acad. Sci. USA 108, 19335–19340 (2011).
Sugita, M. et al. Separate pathways for antigen presentation by CD1 molecules. Immunity 11, 743–752 (1999).
Barral, D.C. et al. CD1a and MHC class I follow a similar endocytic recycling pathway. Traffic 9, 1446–1457 (2008).
Layre, E. et al. A comparative lipidomics platform for chemotaxonomic analysis of Mycobacterium tuberculosis. Chem. Biol. 18, 1537–1549 (2011).
Murphy, G.F., Bhan, A.K., Sato, S., Mihm, M.C. Jr. & Harrist, T.J. A new immunologic marker for human Langerhans cells. N. Engl. J. Med. 304, 791–792 (1981).
Crozat, K. et al. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol. Rev. 234, 177–198 (2010).
Nicolaides, N. Skin lipids: their biochemical uniqueness. Science 186, 19–26 (1974).
Touchstone, J.C. Thin-layer chromatographic procedures for lipid separation. J. Chromatogr. B Biomed. Appl. 671, 169–195 (1995).
Moody, D.B. et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 278, 283–286 (1997).
de Jong, A. et al. CD1c presentation of synthetic glycolipid antigens with foreign alkyl branching motifs. Chem. Biol. 14, 1232–1242 (2007).
Lampe, M.A. et al. Human stratum corneum lipids: characterization and regional variations. J. Lipid Res. 24, 120–130 (1983).
Kellum, R.E. Human sebaceous gland lipids. Analysis by thin-layer chromatography. Arch. Dermatol. 95, 218–220 (1967).
Zajonc, D.M., Elsliger, M.A., Teyton, L. & Wilson, I.A. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 Å. Nat. Immunol. 4, 808–815 (2003).
Manolova, V. et al. Functional CD1a is stabilized by exogenous lipids. Eur. J. Immunol. 36, 1083–1092 (2006).
Mallevaey, T. et al. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity 34, 315–326 (2011).
Rossjohn, J., Pellicci, D.G., Patel, O., Gapin, L. & Godfrey, D.I. Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 12, 845–857 (2012).
Garcia-Alles, L.F. et al. Endogenous phosphatidylcholine and a long spacer ligand stabilize the lipid-binding groove of CD1b. EMBO J. 25, 3684–3692 (2006).
Dieudé, M. et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted gammadelta T cells in the normal murine repertoire. J. Immunol. 186, 4771–4781 (2011).
McCarthy, C. et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J. Exp. Med. 204, 1131–1144 (2007).
Di Nardo, A., Wertz, P., Giannetti, A. & Seidenari, S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm. Venereol. 78, 27–30 (1998).
Motta, S. et al. Ceramide composition of the psoriatic scale. Biochim. Biophys. Acta 1182, 147–151 (1993).
Kircik, L.H., Del Rosso, J.Q. & Aversa, D. Evaluating clinical use of a ceramide-dominant, physiologic lipid-based topical emulsion for atopic dermatitis. J. Clin. Aesthetic Dermatol. 4, 34–40 (2011).
O'Hagan, D.T., Ott, G.S., De Gregorio, E. & Seubert, A. The mechanism of action of MF59–an innately attractive adjuvant formulation. Vaccine 30, 4341–4348 (2012).
Peña-Cruz, V. et al. Extraction of human Langerhans cells: a method for isolation of epidermis-resident dendritic cells. J. Immunol. Methods 255, 83–91 (2001).
Tachi, M. & Iwamori, M. Mass spectrometric characterization of cholesterol esters and wax esters in epidermis of fetal, adult and keloidal human skin. Exp. Dermatol. 17, 318–323 (2008).
Gras, S. et al. The shaping of T cell receptor recognition by self-tolerance. Immunity 30, 193–203 (2009).
Acknowledgements
We thank C. Higgins (Columbia University) for the photo of sebaceous glands; L.L. Tan for technical support for production of the BC2 TCR; and members of the US National Institutes of Health Tetramer Facility for the preparation of CD1a. Supported by the Burroughs Wellcome Fund (D.B.M.), the Mizutani Foundation, the US National Institutes of Health (AR R01048632 and AI 049313 to D.B.M.; K08AI089858 to A.G.K.; Tetramer Facility contract HHSN272201300006C to J.D.A.), the Dermatology Foundation (A.d.J.), the American Skin Association (A.d.J.), the Australian Research Council (S.G.) and the National Health and Medical Research Council of Australia (J.R.).
Author information
Authors and Affiliations
Contributions
A.d.J. and D.B.M. designed research and wrote the manuscript; A.d.J. and T.-Y.C. designed and performed experiments. A.G.K. did CD1a-precipitation assays and contributed to cell-free assays. S.H. did acid stripping and reloading of CD1 eluents for T cell activation, elution and quadrupole time-of-flight mass spectrometry of CD1a ligands; V.P.-C. provided epidermal sheets for lipid extraction; D.T.R. provided thyroid tissue samples; J.D.A. provided biotinylated CD1a monomers; I.V.R. identified alleles encoding TCR α- and β-chains; S.G., R.W.B. and J.R. designed experiments and contributed surface plasmon resonance data; and J.R. assisted in preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Low pH promotes unloading of lipids from CD1a protein.
Plate-bound CD1a protein was treated with citrate buffers of indicated pH. 24h after co-incubation of BC2 T cell line with pH-treated CD1a protein, T cell activation was measured in the supernatant by IFNγ ELISA
Supplementary Figure 4 Electrospray-ionization mass spectrometry of lipids eluted from silica fraction of a TLC plate.
Comparison of collision induced dissociation mass spectrometry profiles with lipid standards
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 1 (PDF 4720 kb)
Rights and permissions
About this article
Cite this article
de Jong, A., Cheng, TY., Huang, S. et al. CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat Immunol 15, 177–185 (2014). https://doi.org/10.1038/ni.2790
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2790
This article is cited by
-
CD1-mediated immune responses in mucosal tissues: molecular mechanisms underlying lipid antigen presentation system
Experimental & Molecular Medicine (2023)
-
Staphylococcal phosphatidylglycerol antigens activate human T cells via CD1a
Nature Immunology (2023)
-
CD1a promotes systemic manifestations of skin inflammation
Nature Communications (2022)
-
Human unconventional T cells in Plasmodium falciparum infection
Seminars in Immunopathology (2020)
-
T cells and the skin: from protective immunity to inflammatory skin disorders
Nature Reviews Immunology (2019)