Abstract
Microbes or danger signals trigger inflammasome sensors, which induce polymerization of the adaptor ASC and the assembly of ASC specks. ASC specks recruit and activate caspase-1, which induces maturation of the cytokine interleukin 1β (IL-1β) and pyroptotic cell death. Here we found that after pyroptosis, ASC specks accumulated in the extracellular space, where they promoted further maturation of IL-1β. In addition, phagocytosis of ASC specks by macrophages induced lysosomal damage and nucleation of soluble ASC, as well as activation of IL-1β in recipient cells. ASC specks appeared in bodily fluids from inflamed tissues, and autoantibodies to ASC specks developed in patients and mice with autoimmune pathologies. Together these findings reveal extracellular functions of ASC specks and a previously unknown form of cell-to-cell communication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Latz, E. et al. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Masumoto, J. et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 274, 33835–33838 (1999).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014).
Cai, X. et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156, 1207–1222 (2014).
Matsushita, K. et al. A splice variant of ASC regulates IL-1β release and aggregates differently from intact ASC. Mediators Inflamm. 2009, 287387 (2009).
Wu, H. Higher-order assemblies in a new paradigm of signal transduction. Cell 153, 287–292 (2013).
Proell, M., Gerlic, M., Mace, P.D., Reed, J.C. & Riedl, S.J. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem. J. 449, 613–621 (2013).
Faustin, B. et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25, 713–724 (2007).
Bergsbaken, T. et al. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Keller, M., Ruegg, A., Werner, S. & Beer, H.-D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008).
Balci-Peynircioglu, B. et al. Expression of ASC in renal tissues of familial Mediterranean fever patients with amyloidosis: postulating a role for ASC in AA type amyloid deposition. Exp. Biol. Med. (Maywood) 233, 1324–1333 (2008).
Stutz, A., Horvath, G.L., Monks, B.G. & Latz, E. ASC speck formation as a readout for inflammasome activation. Methods Mol. Biol. 1040, 91–101 (2013).
Jakobs, C., Bartok, E., Kubarenko, A., Bauernfeind, F. & Hornung, V. Immunoblotting for active caspase-1. Methods Mol. Biol. 1040, 103–115 (2012).
Lima-Junior, D.S. et al. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat. Med. 19, 909–915 (2013).
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 (2008).
Sheedy, F.J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Westwell-Roper, C., Dunne, A., Kim, M.L., Verchere, C.B. & Masters, S.L. Activating the NLRP3 inflammasome using the amyloidogenic peptide IAPP. Methods Mol. Biol. 1040, 9–18 (2013).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Martinon, F. et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Masters, S.L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Duewell, P., Duewell, P., Latz, E. & Latz, E. Assessment and quantification of crystal-induced lysosomal damage. Methods Mol. Biol. 1040, 19–27 (2013).
Aguzzi, A. Cell biology: Beyond the prion principle. Nature 459, 924–925 (2009).
Hofmann, J.P. et al. Cell-to-cell propagation of infectious cytosolic protein aggregates. Proc. Natl. Acad. Sci. USA 110, 5951–5956 (2013).
Aguzzi, A., Nuvolone, M. & Zhu, C. The immunobiology of prion diseases. Nat. Rev. Immunol. 13, 888–902 (2013).
Aguzzi, A. & Falsig, J. Prion propagation, toxicity and degradation. Nat. Neurosci. 15, 936–939 (2012).
Wang, X. et al. The molecular basis of functional bacterial amyloid polymerization and nucleation. J. Biol. Chem. 283, 21530–21539 (2008).
Holmes, B.B. & Diamond, M.I. Cellular mechanisms of protein aggregate propagation. Curr. Opin. Neurol. 25, 721–726 (2012).
Sutterwala, F.S. et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204, 3235–3245 (2007).
De Nardo, D., De Nardo, C.M. & Latz, E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am. J. Pathol. 184, 42–54 (2013).
Botelho, F.M. et al. IL-1α/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLoS ONE 6, e28457 (2011).
Sci, E. et al. A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J. Allergy Clin. Immunol. 131, 752–762 (2013).
Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835 (2006).
Leiss, H. et al. Pristane-induced lupus as a model of human lupus arthritis: evolvement of autoantibodies, internal organ and joint inflammation. CORD Conf. Proc. 22, 778–792 (2012).
Hou, F. et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011).
Li, J. et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 (2012).
Wu, B. et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 152, 276–289 (2012).
Bakele, M. et al. Localization and functionality of the inflammasome in neutrophils. J. Biol. Chem. 289, 5320–5329 (2014).
Adamczak, S. et al. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome. J. Neurosurg. 117, 1119–1125 (2012).
Meissner, F., Molawi, K. & Zychlinsky, A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat. Immunol. 9, 866–872 (2008).
de Rivero Vaccari, J.P. et al. A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 28, 3404–3414 (2008).
de Rivero Vaccari, J.P. et al. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J. Cereb. Blood Flow Metab. 29, 1251–1261 (2009).
Stewart, C.R. et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 (2010).
Fernandes-Alnemri, T., Fernandes-Alnemri, T., Alnemri, E.S. & Alnemri, E.S. Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol. 442, 251–270 (2008).
Kastenmüller, W., Torabi-Parizi, P., Subramanian, N., Lämmermann, T. & Germain, R.N.A. Spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 150, 1235–1248 (2012).
Acknowledgements
We thank C. Tiberi and P.-I. Chiang for technical support. Supported by Deutsche Forschungsgemeinschaft (SFB670 to E.L.), the US National Institutes of Health (R01-HL093262, R01-HL112661 and R01-AI083713 to E.L.), the Alexander von Humboldt Foundation (B.S.F.), intramural BONFOR research support at the University of Bonn (B.S.F.), the European Research Council (Advanced Grant to A.A.), the European Union (NEURINOX to A.A.), the Swiss National Foundation (Sinergia grant to A.A.), the Novartis Research Foundation (A.A.), the Clinical Research Priority Programs “Small RNAs” and “Mechanisms of Human Hemato-Lymphatic Diseases” of the University of Zurich (A.A.), the ImmunoSensation cluster of Excellence in Bonn (E.L.) and the German Center for Infection Research (E.L.).
Author information
Authors and Affiliations
Contributions
B.S.F., L.B. and W.K. designed and did experiments and analyzed data; J.M.R., A.S., G.E., M.S.M., B.G.M., P.B. and S.H. did experiments; C.B., M.N., S.R.M., A.A.-A., S.H. and A.A. did and analyzed data from electron microscopy and cryo–electron microscopy; D.D.N., T.E., P.B., A.M.-R., A.A. and W.K. analyzed data and provided critical suggestions and discussions throughout the study; S.Z. provided BALF samples from patients with COPD; L.B., M.F. and R.E.S. provided serum samples from patients with autoimmune disease; B.J., A.G.J. and P.M.H. provided the mouse model of COPD; B.S.F. and E.L. designed the study; and B.S.F., D.D.N. and E.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
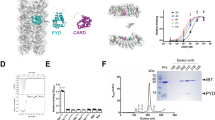
Supplementary Figure 1 ASC forms speck-like clusters in various cells.
(a) Confocal imaging of unstimulated (–) or LPS-primed (200 pg/ml), nigericin-activated (10 μM) human PBMCs (upper panel) or THP-1 monocytes (LPS 1 μg/ml, nigericin 10 μM, lower panel). Scale bars: 22 μm. (b) Flow charge and flow cytometry analysis and fluorescence imaging of ASC-mCerulean specks that were spiked into supernatants of LPS-primed (1 μg/ml), nigericin-activated (10 μM) THP-1 monocytes. Data is representative of two independent experiments.
Supplementary Figure 2 Molecular composition of extracellular ASC specks.
(a) Immunoblot of ASC and NLRP3 in ASC specks purified from cell-free supernatants or from whole cell lysates of LPS-primed, ATP activated Wt, Pycard–/–, Casp1–/– or Nlrp3–/– iMøs. (b) Flow cytometry analysis showing in vitro assembly of ASC-mCerulean specks from cytosolic lysates (S100) of untreated ASC-mCerulean expressing cells. (c) Flow cytometry analysis of in vitro-assembled ASC specks prepared as in b, that were left unstained (Uns) or stained with anti-ASC or anti-Caspase1 Abs followed by staining with A488-direclty conjugated secondary Abs. Samples were also stained with the caspase-1 inhibitor z-YVAD-FMK-FITC and compared to ASC specks generated from Casp1–/– iMøs. Beads with defined sizes (μm) were used as reference. (d) Flow cytometry analysis showing the purification with a 50% percoll cushion and immunoblot for ASC, NLRP3 and Caspase-1 in in vitro-assembled ASC-mCerulean specks prepared as in b. (e) Confocal imaging of LPS-primed and nigericin-activated WT THP-1 monocytes. Cells were fixed and stained with anti-ASC or anti-NLRP3 Abs coupled to fluorochrome-conjugated secondary Abs. Arrows show intra- and extracellular specks containing both endogenous ASC and NLRP3 proteins. Scale bar: 3.2 μm. (f) Confocal imaging of LPS-primed NLRP3-mCitrine expressing iMøs left untreated (UT) or activated with nigericin (10 μm). Cells were fixed and stained with anti-ASC Abs followed by staining with fluorescence-conjugated secondary Abs. The mean fluorescence intensity (MFI) of ASC or NLRP3 was compared in the cytosol (soluble) and on the specks. Data are from one representative out of two (a-f) independent experiments.
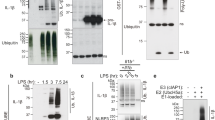
Supplementary Figure 3 ASC specks recruit pro-caspase-1 from cell-free supernatants.
(a) Immunoblot for IL-1β and Caspase-1 in cell-free supernatants from untreated or LPS-primed (500 ng/ml, 3h), ATP activated (2.5 mM) iMøs before and after addition of fluorescent recombinant ASC-mCerulean specks generated from WT or Casp1–/– iMøs. (b) Immunoblot for Caspase-1 and GFP in in recombinant ASC-mCerulean specks from WT or Casp1–/– iMøs that were incubated with cell-free supernatants of unstimulated (None), or LPS-primed and ATP, or nigericin-activated iMøs. Data are from one representative of two independent experiments.
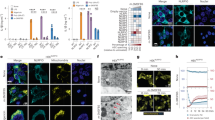
Supplementary Figure 4 ASC has 'prionoid' features.
(a) Cryo-EM of in vitro-assembled ASC-mCerulean specks. Scale bars: 100 nm and 200 nm. (b) Time-lapse confocal imaging of the in vitro nucleation of soluble ASC-mCherry (red) by ASC-mCerulean specks (green). Scale bar: 4.4 μm. Graph shows mean fluorescence intensity over time of ASC-mCerulean specks after addition of soluble ASC-mCherry. (c) Rendered 3D confocal image of z-stack sections of ASC-mCerulean specks after incubation with soluble ASC-mCherry. (d) Flow cytometry of pre-assembled ASC-mCerulan specks incubated with soluble ASC-mCherry for the indicated time. (e) Confocal imaging of the nucleation of soluble ASC-mCherry, or FC-mCherry protein by ASC-mCerulean specks. Images were recorded every 30 seconds at 63X magnification. Data are from one representative of two (a,d,e) or three (b,c) independent experiments.
Supplementary Figure 5 ASC specks are resistant to proteases in vivo.
(a) Confocal images showing whole mount staining of ears from LysMgfp/+ transgenic mice at 24, 48, 72 or 96 hours post injection with 1 μg of ASC-mCerulean specks in 10 μl of PBS. (b) Confocal images of whole skin mount of wild-type mice injected in the ear with 1μg of ASC-mCerulean specks. Tissues were stained with anti-ASC (AL177) and anti-Rb Alexa Fluor 568. Scale bars: 20 μm. Data show one representative of two independent experiments.
Supplementary Figure 6 Autoimmune serum contains antibodies to ASC specks.
(a) ELISA of anti-ASC specks Abs in sera from autoimmune patients (n = 14), or healthy donors (n = 7). (b) ELISA of anti-ASC specks Abs in sera from autoimmune (n = 10), or control (n = 10) mice. ELISA plates were coated with 50 μg/ml of ASC specks or PBS. Anti-ASC specks Abs in sera were detected with specific anti-human or anti-mouse HRP conjugated secondary Abs. The absorbance (OD) at 450 nm was normalized against the OD obtained in wells coated with PBS and assayed with anti-human or mouse-HRP Abs. The reactivity of secondary Abs against ASC specks without human or mouse sera is shown.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 9670 kb)
Supplementary Video 1
Time-lapse confocal imaging of ATP-activated (5 mM) inflammasome reporter iMΦs expressing ASC-mCerulean (green). Images were recorded every 5 min. Scale bar: 48 μm. (MOV 9434 kb)
Supplementary Video 2
Time-lapse fluorescence imaging of LPS-primed and nigericin-activated THP-1 cells expressing ASC-mCerulean (green). Nuclei (blue) was stained with Draq5. Images were recorded every 5 sec. Scale bar: 25 μm. (MOV 1128 kb)
Supplementary Video 3
Time-lapse confocal imaging of LPS-primed and nigericin-activated THP-1 cells expressing ASC-mCerulean (red). Plasma membrane (green) was stained with CTxB-Alexa Fluor 555. Images were recorded every 5 sec. Scale bar: 25 μm. (MOV 5148 kb)
Supplementary Video 4
Time-lapse confocal imaging of LPS-primed and nigericin-activated THP-1 cells expressing ASC-mCerulean (green) in the presence of propidium iodide (red). Nuclei (blue) was stained with Draq 5. Images were recorded every 5 min. Scale bar: 30 μm. (MOV 4348 kb)
Supplementary Video 5
Time-lapse confocal imaging of ATP activated ASCmCerulean expressing iMΦs in the presence of 1 μg of Alexa Fluor 555-conjugated anti-GFP mAb. Images were recorded every 5.5 min, scale bar: 80 μm. (MOV 4434 kb)
Supplementary Video 6
Time-lapse confocal imaging of ASC-mCerulean specks-mediated nucleation of soluble ASC-mCherry. ASC-mCerulean specks were seeded into glass-bottom dishes and cytosols from ASC-mCherry expressing cells were added during imaging. Images were recorded every 3 seconds. (MP4 11570 kb)
Rights and permissions
About this article
Cite this article
Franklin, B., Bossaller, L., De Nardo, D. et al. The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat Immunol 15, 727–737 (2014). https://doi.org/10.1038/ni.2913
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2913
This article is cited by
-
Biological and clinical roles of IL-18 in inflammatory diseases
Nature Reviews Rheumatology (2024)
-
Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets
Nature Reviews Drug Discovery (2024)
-
Exercise suppresses neuroinflammation for alleviating Alzheimer’s disease
Journal of Neuroinflammation (2023)
-
Characterization and inhibition of inflammasome responses in severe and non-severe asthma
Respiratory Research (2023)
-
Microglial function, INPP5D/SHIP1 signaling, and NLRP3 inflammasome activation: implications for Alzheimer’s disease
Molecular Neurodegeneration (2023)