Abstract
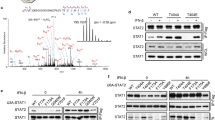
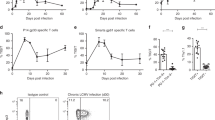
Suppressor of cytokine signaling 1 (SOCS1) is a critical regulator of cytokine signaling and immune responses. SOCS1-deficient mice develop severe inflammatory disease, but are very resistant to viral infections. Using neutralizing antibody to type I interferon (IFN-α and IFN-β) and mice deficient in interferon-γ or type I interferon receptor components (IFNAR1 or IFNAR2), we demonstrate here that SOCS1 deficiency amplified type I interferon antiviral and proinflammatory actions independently of interferon-γ. The mechanism of the suppression of type I interferon responses by SOCS1 was distinct from that of other cytokines. SOCS1 associated with and regulated IFNAR1- but not IFNAR2-specific signals, abrogating tyrosine phosphorylation of transcription factor STAT1 and reducing the duration of antiviral gene expression. Thus, SOCS1 is an important in vivo inhibitor of type I interferon signaling and contributes to balancing its beneficial antiviral versus detrimental proinflammatory effects on innate immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gutterman, J.U. Cytokine therapeutics: lessons from interferon-α. Proc. Natl. Acad. Sci. USA 91, 1198–1205 (1994).
Issac, A. & Lindemann, J. Virus interference I: the interferons. Proc. Royal Soc. Lond. B 147, 258–267 (1957).
Pestka, S., Langer, J.A., Zoon, K.C. & Samuel, C.E. Interferons and their actions. Annu. Rev. Biochem. 56, 727–777 (1987).
Hertzog, P.J. et al. A gene on human chromosome 21 located in the region 21q22.2 to 22q22.3 encodes a factor necessary for signal transduction and antiviral response to type I interferons. J. Biol. Chem. 269, 14088–14093 (1994).
Weissmann, C. & Weber, H. The interferon genes. Prog. Nucleic Acid Res. Mol. Biol. 33, 251–300 (1986).
Oritani, K., Kincade, P.W., Zhang, C., Tomiyama, Y. & Matsuzuma, Y. Type I interferons and limitin: a comparison of structures, receptors and functions. Cytokine Growth Factor Rev. 12, 337–348 (2001).
Le Bon, A. et al. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14, 461–470 (2001).
Bach, E.A., Aguet, M. & Schreiber, R.D. The IFN-γ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 15, 563–591 (1997).
Godfrey, D.I., Hammond, K.J., Poulton, L.D., Smythe, M.J. & Baxter, A.G. NKT cells: facts, functions and fallacies. Immunol. Today 21, 573–583 (2000).
Darnell, J.E., Kerr, I.M. & Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1421 (1994).
Stark, G.R., Kerr, I.M., Williams, B.R., Silverman, R.H. & Schreiber, R.D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Gresser, I. How does interferon inhibit tumour growth? Phil. Trans. R. Soc. Lond. B 299, 69–76 (1982).
Neel, B.G. Role of phosphatases in lymphocyte activation. Curr. Opin. Immunol. 9, 405–420 (1997).
Irie-Sasaki, J. et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signaling. Nature 409, 349–354 (2001).
Starr, R. et al. A family of cytokine-inducible inhibitors of signaling. Nature 387, 917–921 (1997).
Endo, T.A. et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387, 921–924 (1997).
Naka, T. et al. Structure and function of a new STAT-induced STAT inhibitor. Nature 387, 924–929 (1997).
Yoshimura, A. et al. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin-3 and erythropoietin receptors. EMBO J. 14, 2816–2826 (1995).
Hilton, D.J. et al. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc. Natl. Acad. Sci. USA 95, 114–119 (1998).
Nicholson, S.E. et al. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 18, 375–385 (1999).
Sasaki, A. et al. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 4, 339–351 (1999).
Zhang, J.-G. et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc. Natl. Acad. Sci. USA 96, 2071–2076 (1999).
Nicholson, S.E. et al. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc. Natl. Acad. Sci. USA 97, 6493–6498 (2000).
Yasukawa, H. et al. The JAK binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18, 1309–1320 (1999).
Kile, B.T. et al. The SOCS box: a tale of destruction and degradation. Trends Biochem. Sci. 27, 235–241 (2002).
Adams, T.E. et al. Growth hormone preferentially induces the rapid, transient expression of SOCS3, a novel inhibitor of cytokine signaling. J. Biol. Chem. 273, 1285–1287 (1998).
Sakamoto, H. et al. A Janus kinase inhibitor, JAB, is an interferon-γ inducible gene and confers resistance to interferons. Blood 92, 1668–1676 (1998).
Bjorbaek, C., Elmquist, J.K., Frantz, J.D., Shoelson, S.E. & Flier, J.S. Identification of SOCS3 as a potential mediator of central leptin resistance. Mol. Cell 1, 619–625 (1998).
Starr, R. et al. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc. Natl. Acad. Sci. USA 95, 14395–14399 (1998).
Naka, T. et al. Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc. Natl. Acad. Sci. USA 95, 15577–15582 (1998).
Alexander, W.S. et al. SOCS 1 is a critical inhibitor of interferon γ signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98, 1–20 (1999).
Marine, J.C. et al. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 98, 609–616 (1999).
Brysha, M. et al. Suppressor of cytokine signaling 1 attenuates the duration of interferon gamma signal transduction in vitro and in vivo. J. Biol. Chem. 276, 22086–22089 (2001).
Hertzog, P.J., Emery, P., Cheetham, B.F., Mackay, I.R. & Linnane, A.W. Interferons in rheumatoid arthritis: Alterations in production and response related to disease activity. Clin. Immunol. Immunopathol. 48, 192–201 (1988).
Song, M.M. & Shuai, K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J. Biol. Chem. 273, 35056–35062 (1998).
Kubo, M., Hanada, T. & Yoshimura, A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 4, 1169–1176 (2003).
Bullen, D.V., Darwiche, R., Metcalf, D., Handman, E. & Alexander, W.S. Neutralisation of interferon-γ in neonatal Socs1−/− mice prevents fatty degeneration of the liver but not subsequent fatal inflammatory disease. Immunology 104, 92–98 (2001).
Metcalf, D., Mifsud, S., Di Rago, L. & Alexander, W.S. The lethal effects of transplantation of Socs1−/− bone marrow cells into irradiated adult syngeneic recipients. Proc. Natl. Acad. Sci. USA 100, 8436–8441 (2003).
Dalton, D.K. et al. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259, 1739–1742 (1993).
Biron, C.A. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr. Opin. Immunol. 6, 530–538 (1994).
Müller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994).
Hwang, S.Y. et al. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons α and β and alters macrophage responses. Proc. Natl. Acad. Sci. USA 92, 11284–11288 (1995).
Eyles, J.L., Metcalf, D., Grusby, M.J., Hilton, D.J. & Starr, R. Negative regulation of interleukin-12 signaling by suppressor of cytokine signaling 1. J. Biol. Chem. 277, 43735–43740 (2002).
Hamilton, J.A., Whitty, G.A., Kola, I. & Hertzog, P.J. Endogenous IFNα/β suppresses colony-stimulating factor (CSF)-1-stimulated macrophage DNA synthesis and mediates inhibitory effects of lipopolysaccharide and TNFα. J. Immunol. 156, 2553–2557 (1996).
Hertzog, P.J., O'Neill, L.A. & Hamilton, J.A. The interferon in TLR signaling: more than just antiviral. Trends Immunol. 24, 534–539 (2003).
Takaoka, A. et al. Cross talk between interferon-γ and –α/β signaling components in caveolar membrane domains. Science 288, 2357–2360 (2000).
Cornish, A.L. et al. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other γc-dependent cytokines in peripheral T cells. J. Biol. Chem. 278, 22755–22761 (2003).
Chong, M.M. et al. Suppressor of cytokine signaling-1 is a critical regulator of interleukin-7-dependant CD8+ T cell differentiation. Immunity 18, 475–487 (2003).
Sweet, M.J. et al. A novel pathway regulating lipopolysaccharide-Induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J. Immunol. 166, 6633–6639 (2001).
Hertzog, P.J. Isolation of embryonic fibroblasts and their use in the in vitro characterization of gene function. Methods Mol. Biol. 158, 205–215 (2001).
Dunn, G.P. et al. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 6, 722–729 (2005).
Wilson-Annon, J. et al. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J. Cell. Biol. 162, 877–887 (2003).
Acknowledgements
Supported by the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fenner, J., Starr, R., Cornish, A. et al. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol 7, 33–39 (2006). https://doi.org/10.1038/ni1287
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1287
This article is cited by
-
Suppressor of cytokine signaling-1 mimetic peptides attenuate lymphocyte activation in the MRL/lpr mouse autoimmune model
Scientific Reports (2021)
-
Influenza a virus antagonizes type I and type II interferon responses via SOCS1-dependent ubiquitination and degradation of JAK1
Virology Journal (2020)
-
The Neuro-Immune-Regulators (NIREGs) Promote Tissue Resilience; a Vital Component of the Host’s Defense Strategy against Neuroinflammation
Journal of Neuroimmune Pharmacology (2018)
-
Oxymatrine Sensitizes the HaCaT Cells to the IFN-γ Pathway and Downregulates MDC, ICAM-1, and SOCS1 by Activating p38, JNK, and Akt
Inflammation (2018)
-
NOD1 modulates IL-10 signalling in human dendritic cells
Scientific Reports (2017)