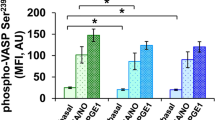
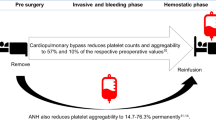
Abstract
Rapid chilling causes glycoprotein-Ib (GPIb) receptors to cluster on blood platelets. Hepatic macrophage β2 integrin binding to β-N-acetylglucosamine (β-GlcNAc) residues in the clusters leads to rapid clearance of acutely chilled platelets after transfusion. Although capping the β-GlcNAc moieties by galactosylation prevents clearance of short-term–cooled platelets, this strategy is ineffective after prolonged refrigeration. We report here that prolonged refrigeration increased the density and concentration of exposed galactose residues on platelets such that hepatocytes, through Ashwell-Morell receptor binding, become increasingly involved in platelet removal. Macrophages rapidly removed a large fraction of transfused platelets independent of their storage conditions. With prolonged platelet chilling, hepatocyte-dependent clearance further diminishes platelet recovery and survival after transfusion. Inhibition of chilled platelet clearance by both β2 integrin and Ashwell-Morell receptors may afford a potentially simple method for storing platelets in the cold.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Becker, G.A, Tuccelli, M., Kunicki, T., Chalos, M.K. & Aster, R.H. Studies of platelet concentrates stored at 22 °C and 4 °C. Transfusion 13, 61–68 (1973).
Hoffmeister, K.M. et al. The clearance mechanism of chilled blood platelets. Cell 112, 87–97 (2003).
Murphy, S. & Gardner, F. Effect of storage temperature on maintenance of platelet viability–deleterious effect of refrigerated storage. N. Engl. J. Med. 280, 1094–1098 (1969).
Kuehnert, M.J. et al. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion 41, 1493–1499 (2001).
Blajchman, M. Bacterial contamination of blood products. Transfus. Apher. Sci. 24, 245 (2001).
Sullivan, M.T., Cotten, R., Read, E.J. & Wallace, E.L. Blood collection and transfusion in the United States in 2001. Transfusion 3, (2001).
McCullough, J. Transfusion Medicine 2011–2068 (Lipincott Wilkins & Williams, Philadelphia, 2003).
Webert, K.E. et al. Proceedings of a Consensus Conference: pathogen inactivation-making decisions about new technologies. Transfus. Med. Rev. 22, 1–34 (2008).
Hoffmeister, K.M. et al. Glycosylation restores survival of chilled blood platelets. Science 301, 1531–1534 (2003).
Wandall, H.H. et al. Galactosylation does not prevent the rapid clearance of long-term, 4 °C–stored platelets. Blood 111, 3249–3256 (2008).
McGill, M. Temperature cycling preserves platelet shape and enhances in vitro test scores during storage at 4 degrees. J. Lab. Clin. Med. 92, 971–982 (1978).
McGill, M. Platelet storage by temperature cycling. Prog. Clin. Biol. Res. 28, 119–139 (1978).
Valeri, C.R., Giorgio, A., Macgregor, H. & Ragno, G. Circulation and distribution of autotransfused fresh, liquid-preserved and cryopreserved baboon platelets. Vox Sang. 83, 347–351 (2002).
Alves-Rosa, F. et al. Macrophage depletion following liposomal-encapsulated clodronate (LIP-CLOD) injection enhances megakaryocytopoietic and thrombopoietic activities in mice. Br. J. Haematol. 121, 130–138 (2003).
van Rooijen, N. & van Nieuwmegen, R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate: an enzyme-histochemical study. Cell Tissue Res. 238, 355–358 (1984).
van Rooijen, N. van Kesteren-Hendrikx, E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 373, 3–16 (2003).
Josefsson, E.C., Gebhard, H.H., Stossel, T.P., Hartwig, J.H. & Hoffmeister, K.M. The macrophage αMβ2 integrin αM lectin domain mediates the phagocytosis of chilled platelets. J. Biol. Chem. 280, 18025–18032 (2005).
Grewal, P.K. et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat. Med. 14, 648–655 (2008).
Berndt, M.C. et al. Purification and preliminary characterization of the glycoprotein Ib complex in the human platelet membrane. Eur. J. Biochem. 151, 637–649 (1985).
Lopez, J.A. et al. Cloning of the α chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich α 2-glycoprotein. Proc. Natl. Acad. Sci. USA 84, 5615–5619 (1987).
Tsuji, T. et al. The carbohydrate moiety of human platelet glycocalicin. J. Biol. Chem. 258, 6335–6339 (1983).
Tsuji, T. & Osawa, T. The carbohydrate moiety of human platelet glycocalicin: the structures of the major Asn-linked sugar chains. J. Biochem. 101, 241–249 (1987).
Ezzell, R., Kenney, D., Egan, S., Stossel, T. & Hartwig, J. Localization of the domain of actin-binding protein that binds to membrane glycoprotein Ib and actin in human platelets. J. Biol. Chem. 263, 13303–13309 (1988).
Rensen, P.C. et al. Detemination of the upper size limit for uptake and processing of ligands by the asialoglycoprotein receptor on hepacytes in vitro and in vivo. J. Biol. Chem. 276, 37577–37584 (2001).
Ozaki, K., Lee, R.T., Lee, Y.C. & Kawasaki, T. The differences in structural specificity for recognition and binding between asialoglycoprotein receptors of liver and macrophages. Glycoconj. J. 12, 268–274 (1995).
Schlepper-Schäfer, J. et al. Endocytosis via galactose receptors in vivo. Ligand size directs uptake by hepatocytes and/or liver macrophages. Exp. Cell Res. 165, 494–506 (1986).
Kolb-Bachofen, V., Schlepper-Schafer, J., Roos, P., Hulsmann, D. & Kolb, H. GalNAc/Gal-specific rat liver lectins: their role in cellular recognition. Biol. Cell 51, 219–226 (1984).
Mason, K.D. et al. Programmed anuclear cell death delimits platelet life span. Cell 128, 1173–1186 (2007).
Ellies, L.G. et al. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc. Natl. Acad. Sci. USA 99, 10042–10047 (2002).
Hoffmeister, K.M. et al. Mechanisms of cold-induced platelet actin assembly. J. Biol. Chem. 276, 24751–24759 (2001).
Crowe, J.H. et al. Stabilization of membranes in human platelets freeze-dried with trehalose. Chem. Phys. Lipids 122, 41–52 (2003).
Gousset, K., Tsvetkova, N.M., Crowe, J.H. & Tablin, F. Important role of raft aggregation in the signaling events of cold-induced platelet activation. Biochim. Biophys. Acta 1660, 7–15 (2004).
Leidy, C. et al. Lipid phase behavior and stabilization of domains in membranes of platelets. Cell Biochem. Biophys. 40, 123–148 (2004).
Bergmeier, W. et al. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro–aged or –injured mouse platelets. Blood 102, 4229–4235 (2003).
Korrel, S. et al. Identification of a tetrasialylated monofucosylated tetraantennary N-linked carbohydrate chain in human platelet glycocalicin. FEBS Lett. 228, 321–326 (1988).
Tsuji, T. & Osawa, T. The carbohydrate moiety of human platelet glycocalicin: the structures of the major Asn-linked sugar chains. J. Biochem. 101, 241–249 (1987).
Korrel, S.A. et al. Structural studies on the O-linked carbohydrate chains of human platelet glycocalicin. Eur. J. Biochem. 140, 571–576 (1984).
Ward, C., Andrews, R., Smith, A. & Berndt, M. Mocarhagin, a novel cobra venom metalloproteinase, cleaves the platelet von Willebrandt factor receptor glycoprotein Ibα. Identification of the sulfated tyrosine/anionic sequence Tyr-276-Glu-282 of glycoprotein Ibα as a binding site for von Willebrandt factor and α-thrombin. Biochemistry 35, 4929–4938 (1996).
Bergmeier, W. et al. Tumor necrosis factor-α–converting enzyme (ADAM17) mediates GPIbα shedding from platelets in vitro and in vivo. Circ. Res. 95, 677–683 (2004).
Denis, C.V., Christophe, O.D., Oortwijn, B.D. & Lenting, P.J. Clearance of von Willebrand factor. Thromb. Haemost. 99, 271–278 (2008).
Reimers, H.J., Greenberg, J., Cazenave, J.P., Packham, M.A. & Mustard, J.F. Experimental modification of platelet survival. Adv. Exp. Med. Biol. 82, 231–233 (1977).
Wahrenbrock, M.G. & Varki, A. Multiple hepatic receptors cooperate to eliminate secretory mucins aberrantly entering the bloodstream: are circulating carcer mucins the “tip of the iceberg”? Cancer Res. 66, 2433–2441 (2006).
Barber, A.J. & Jamieson, G.A. Platelet collagen adhesion characterization of collagen glucosyltransferase of plasma membranes of human blood platelets. Biochim. Biophys. Acta 252, 533–545 (1971).
Bauvois, B. et al. Membrane glycoprotein IIb is the major endogenous acceptor for human platelet ectosialyltransferase. FEBS Lett. 125, 277–281 (1981).
Soukharev, S., Miller, J.L. & Sauer, B. Segmental genomic replacement in embryonic stem cells by double lox targeting. Nucleic Acids Res. 27, e21 (1999).
Ishibashi, S., Hammer, R.E. & Herz, J. Asialoglycoprotein receptor deficiency in mice lacking the minor receptor subunit. J. Biol. Chem. 269, 27803–27806 (1994).
Prior, I.A., Parton, R.G. & Hancock, J.F. Observing cell surface signaling domains using electron microscopy. Sci. STKE 2003, PL9 (2003).
Acknowledgements
We thank S. Nayeb-Hashemi for excellent technical assistance, H. Clausen for helpful discussions and F. Maignen for statistical expertise. This work was supported by US National Institutes of Health grant PO1 HL056949 (to J.H.H. and K.M.H.) and grant HL089224 (to K.M.H.); The Pew Scholars Award to K.M.H.; PO1HL57345 (to J.D.M.); Howard Hughes Medical Institute to J.D.M.; and The Swedish Medical Research Council, Göteborg University Jubileums stipend to V.R. and K.M.H. V.R. and H.H.W. received sponsored research support form ZymeQuest, Inc. We obtained macros for K-function analysis from J. Hancock at the Institute for Molecular Bioscience, University of Queensland.
Author information
Authors and Affiliations
Contributions
V.R. wrote the manuscript, planned and performed experiments, data analysis and presentation of all figures; P.K.G. provided Asgr-knockout mice and performed experiments; H.H.W. designed research; E.C.J. performed and analyzed experiments; A.L.S. designed research; G.L. designed research; J.D.M. provided Asgr-knockout mice, laboratory space and equipment; J.H.H. wrote the manuscript and performed experiments; K.M.H. designed research, wrote the manuscript and performed experiments.
Corresponding author
Ethics declarations
Competing interests
H.H.W. and K.M.H. are consultants for ZymeQuest. H.H.W. has stock options in ZymeQuest. H.H.W., K.M.H. and V.R. received sponsored research support form ZymeQuest.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Methods (PDF 524 kb)
Rights and permissions
About this article
Cite this article
Rumjantseva, V., Grewal, P., Wandall, H. et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med 15, 1273–1280 (2009). https://doi.org/10.1038/nm.2030
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2030
This article is cited by
-
Platelets from 13-lined ground squirrels are resistant to cold storage lesions
Journal of Comparative Physiology B (2023)
-
Cold-stored platelets for acute bleeding in cardiac surgical patients: a narrative review
Canadian Journal of Anesthesia/Journal canadien d'anesthésie (2023)
-
The GPIb-IX complex on platelets: insight into its novel physiological functions affecting immune surveillance, hepatic thrombopoietin generation, platelet clearance and its relevance for cancer development and metastasis
Experimental Hematology & Oncology (2022)
-
Oseltamivir as rescue therapy for persistent, chronic, or refractory immune thrombocytopenia: a case series and review of the literature
Journal of Thrombosis and Thrombolysis (2022)
-
Kupffer cell receptor CLEC4F is important for the destruction of desialylated platelets in mice
Cell Death & Differentiation (2021)