Abstract
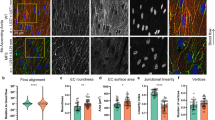
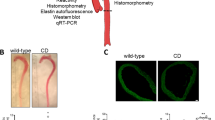
Heritable thoracic aortic aneurysms and dissections (TAAD), including Marfan syndrome (MFS), currently lack a cure, and causative mutations have been identified for only a fraction of affected families. Here we identify the metalloproteinase ADAMTS1 and inducible nitric oxide synthase (NOS2) as therapeutic targets in individuals with TAAD. We show that Adamts1 is a major mediator of vascular homeostasis, given that genetic haploinsufficiency of Adamts1 in mice causes TAAD similar to MFS. Aortic nitric oxide and Nos2 levels were higher in Adamts1-deficient mice and in a mouse model of MFS (hereafter referred to as MFS mice), and Nos2 inactivation protected both types of mice from aortic pathology. Pharmacological inhibition of Nos2 rapidly reversed aortic dilation and medial degeneration in young Adamts1-deficient mice and in young or old MFS mice. Patients with MFS showed elevated NOS2 and decreased ADAMTS1 protein levels in the aorta. These findings uncover a possible causative role for the ADAMTS1–NOS2 axis in human TAAD and warrant evaluation of NOS2 inhibitors for therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dietz, H.C. TGF-β in the pathogenesis and prevention of disease: a matter of aneurysmic proportions. J. Clin. Invest. 120, 403–407 (2010).
Gallo, E.M. et al. Angiotensin-II-dependent TGF-β signaling contributes to Loeys–Dietz syndrome vascular pathogenesis. J. Clin. Invest. 124, 448–460 (2014).
Renard, M. et al. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGF-β signaling in FTAAD. Int. J. Cardiol. 165, 314–321 (2013).
Gillis, E., Van Laer, L. & Loeys, B.L. Genetics of thoracic aortic aneurysm: at the crossroad of transforming-growth-factor-β signaling and vascular smooth muscle cell contractility. Circ. Res. 113, 327–340 (2013).
Habashi, J.P. et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312, 117–121 (2006).
Lim, D.S. et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 103, 789–791 (2001).
Forteza, A. et al. Efficacy of losartan versus atenolol for the prevention of aortic dilation in Marfan syndrome: a randomized clinical trial. Eur. Heart J. 37, 978–985 (2016).
Lacro, R.V. et al. Atenolol versus losartan in children and young adults with Marfan's syndrome. N. Engl. J. Med. 371, 2061–2071 (2014).
Milleron, O. et al. Marfan Sartan: a randomized, double-blind, placebo-controlled trial. Eur. Heart J. 36, 2160–2166 (2015).
Oller, J. et al. C/EBP-β and nuclear factor of activated T cells differentially regulate Adamts1 induction by stimuli associated with vascular remodeling. Mol. Cell. Biol. 35, 3409–3422 (2015).
Luque, A., Carpizo, D.R. & Iruela-Arispe, M.L. ADAMTS1 (METH1) inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 278, 23656–23665 (2003).
Thai, S.N. & Iruela-Arispe, M.L. Expression of ADAMTS1 during murine development. Mech. Dev. 115, 181–185 (2002).
Ren, P. et al. ADAMTS1 and ADAMTS4 levels are elevated in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 95, 570–577 (2013).
Sandy, J.D. et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441–Ala442 bond, a site that is cleaved by recombinant ADAMTS1 and ADAMTS4. J. Biol. Chem. 276, 13372–13378 (2001).
Mittaz, L. et al. Adamts1 is essential for the development and function of the urogenital system. Biol. Reprod. 70, 1096–1105 (2004).
Cohn, R.D. et al. Angiotensin II type 1 receptor blockade attenuates TGF-β-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 13, 204–210 (2007).
Neptune, E.R. et al. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 33, 407–411 (2003).
Pereira, L. et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin 1. Proc. Natl. Acad. Sci. USA 96, 3819–3823 (1999).
Pyeritz, R.E. The Marfan syndrome. Annu. Rev. Med. 51, 481–510 (2000).
Esteban, V. et al. Regulator of calcineurin 1 mediates pathological vascular wall remodeling. J. Exp. Med. 208, 2125–2139 (2011).
Loeys, B.L. et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 37, 275–281 (2005).
Wu, Z., Ruan, Y., Chang, J., Li, B. & Ren, W. Angiotensin II is related to the acute aortic dissection complicated with lung injury through mediating the release of MMP9 from macrophages. Am. J. Transl. Res. 8, 1426–1436 (2016).
Méndez-Barbero, N. et al. A major role for RCAN1 in atherosclerosis progression. EMBO Mol. Med. 5, 1901–1917 (2013).
Förstermann, U. & Sessa, W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 33, 829–837 (2012).
Albrecht, E.W., Stegeman, C.A., Heeringa, P., Henning, R.H. & van Goor, H. Protective role of endothelial nitric oxide synthase. J. Pathol. 199, 8–17 (2003).
Pfeilschifter, J., Eberhardt, W. & Beck, K.F. Regulation of gene expression by nitric oxide. Pflugers Arch. 442, 479–486 (2001).
Tang, C.-H., Lu, D.-Y., Tan, T.-W., Fu, W.-M. & Yang, R.-S. Ultrasound induces hypoxia-inducible factor 1 activation and inducible nitric oxide synthase expression through the integrin–integrin-linked kinase–Akt–mammalian target of rapamycin pathway in osteoblasts. J. Biol. Chem. 282, 25406–25415 (2007).
Partovian, C., Ju, R., Zhuang, Z.W., Martin, K.A. & Simons, M. Syndecan 4 regulates subcellular localization of mTOR complex 2 and Akt activation in a PKC-α-dependent manner in endothelial cells. Mol. Cell 32, 140–149 (2008).
Kleinert, H., Schwarz, P.M. & Förstermann, U. Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 384, 1343–1364 (2003).
Garvey, E.P. et al. 1400W is a slow, tight-binding and highly selective inhibitor of inducible nitric oxide synthase in vitro and in vivo. J. Biol. Chem. 272, 4959–4963 (1997).
Lee, N.V. et al. Fibulin 1 acts as a cofactor for the matrix metalloprotease ADAMTS1. J. Biol. Chem. 280, 34796–34804 (2005).
Shindo, T. et al. ADAMTS1: a metalloproteinase–disintegrin essential for normal growth, fertility, and organ morphology and function. J. Clin. Invest. 105, 1345–1352 (2000).
Le Goff, C. & Cormier-Daire, V. The ADAMTS(L) family and human genetic disorders. Hum. Mol. Genet. 20, R163–R167 (2011).
Hubmacher, D. & Apte, S.S. Genetic and functional linkage between ADAMTS superfamily proteins and fibrillin 1: a novel mechanism influencing microfibril assembly and function. Cell. Mol. Life Sci. 68, 3137–3148 (2011).
Escolano, A. et al. Specific calcineurin targeting in macrophages confers resistance to inflammation via MKP1 and p38. EMBO J. 33, 1117–1133 (2014).
Chen, X., Lu, H., Rateri, D.L., Cassis, L.A. & Daugherty, A. Conundrum of angiotensin II and TGF-β interactions in aortic aneurysms. Curr. Opin. Pharmacol. 13, 180–185 (2013).
Cook, J.R. et al. Dimorphic effects of transforming-growth-factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 35, 911–917 (2015).
Guo, D.C. et al. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am. J. Hum. Genet. 93, 398–404 (2013).
Karimi, A. & Milewicz, D.M. Structure of the elastin–contractile units in the thoracic aorta and how genes that cause thoracic aortic aneurysms and dissections disrupt this structure. Can. J. Cardiol. 32, 26–34 (2016).
Kelwick, R., Desanlis, I., Wheeler, G.N. & Edwards, D.R. The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 16, 113 (2015).
Dan, H.C. et al. Akt-dependent regulation of NF-κB is controlled by mTOR and raptor in association with IKK. Genes Dev. 22, 1490–1500 (2008).
O'Sullivan, S., Medina, C., Ledwidge, M., Radomski, M.W. & Gilmer, J.F. Nitric oxide–matrix metalloproteinase 9 interactions: biological and pharmacological significance—NO and MMP9 interactions. Biochim. Biophys. Acta 1843, 603–617 (2014).
Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 44-46, 224–231 (2015).
Segura, A.M. et al. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan's syndrome. Circulation 98, II331–337; disc. II337–338 (1998).
Biddinger, A., Rocklin, M., Coselli, J. & Milewicz, D.M. Familial thoracic aortic dilatations and dissections: a case control study. J. Vasc. Surg. 25, 506–511 (1997).
Girardi, L.N. & Coselli, J.S. Inflammatory aneurysm of the ascending aorta and aortic arch. Ann. Thorac. Surg. 64, 251–253 (1997).
Roth, M., Lemke, P., Bohle, R.M., Klovekorn, W.P. & Bauer, E.P. Inflammatory aneurysm of the ascending thoracic aorta. J. Thorac. Cardiovasc. Surg. 123, 822–824 (2002).
Gao, Y. et al. A disintegrin and metalloproteinase with thrombospondin motif 1 (ADAMTS1) expression increases in acute aortic dissection. Sci. China Life Sci. 59, 59–67 (2016).
Johanning, J.M. et al. Nitric oxide in experimental aneurysm formation: early events and consequences of nitric oxide inhibition. Ann. Vasc. Surg. 16, 65–72 (2002).
Johanning, J.M., Franklin, D.P., Han, D.C., Carey, D.J. & Elmore, J.R. Inhibition of inducible nitric oxide synthase limits nitric oxide production and experimental aneurysm expansion. J. Vasc. Surg. 33, 579–586 (2001).
Kuhlencordt, P.J. et al. Accelerated atherosclerosis, aortic aneurysm formation and ischemic heart disease in apolipoprotein E and endothelial nitric oxide synthase double-knockout mice. Circulation 104, 448–454 (2001).
Lee, J.K., Borhani, M., Ennis, T.L., Upchurch, G.R. Jr. & Thompson, R.W. Experimental abdominal aortic aneurysms in mice lacking expression of inducible nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 21, 1393–1401 (2001).
Zhang, J. et al. Inducible nitric oxide synthase is present in human abdominal aortic aneurysm and promotes oxidative vascular injury. J. Vasc. Surg. 38, 360–367 (2003).
Fukuda, S. et al. Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase. Circulation 101, 2532–2538 (2000).
Sadamasa, N., Nozaki, K. & Hashimoto, N. Disruption of gene for inducible nitric oxide synthase reduces progression of cerebral aneurysms. Stroke 34, 2980–2984 (2003).
Shores, J., Berger, K.R., Murphy, E.A. & Pyeritz, R.E. Progression of aortic dilatation and the benefit of long-term β-adrenergic blockade in Marfan's syndrome. N. Engl. J. Med. 330, 1335–1341 (1994).
De Backer, J. et al. Marfan syndrome and related heritable thoracic aortic aneurysms and dissections. Curr. Pharm. Des. 21, 4061–4075 (2015).
Judge, D.P. et al. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J. Clin. Invest. 114, 172–181 (2004).
Laubach, V.E., Shesely, E.G., Smithies, O. & Sherman, P.A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. USA 92, 10688–10692 (1995).
Acknowledgements
We thank B. Ibañez and G. Egea for reagents, S. Bartlett for English language editing, A.G. Arroyo, S. Lamas, J. Alegre-Cebollada and J. Ruiz-Cabello for critical reading of the manuscript and advice, and S. Pocock and J. Vazquez for advice on statistics. We also thank the CNIC histology facility, C. Velasco, A.V. Alonso and L. Flores for technical support. CNIC is supported by the Spanish Ministerio de Economía, Industria y Competitividad (MINECO) and the Pro-CNIC Foundation and is a Severo Ochoa Center of Excellence (MINECO award SEV-2015-0505). Support was also provided by grants from MINECO (grants SAF2013-45258P (M.R.C.), SAF2012-34296 (J.M.R.) and SAF2015-636333R (J.M.R.)), Fundacion La Marato (TV3 grants 20151331 (J.M.R.) and 20151330 (A.E.)), CSIC (M.R.C.), the CIBERCV of Ministerio de Sanidad (grant CB16/11/00264; J.M.R.) and the Red de Investigación Cardiovascular (RIC) of Ministerio de Sanidad (grants RD12/0042/0022 (J.M.R.), RD12/0042/0021 (A.E.), RD12/0042/0024 (M.S.), RD12/0042/0056 (J.L.J.-B.) and RD12/0042/0018 (J.F.N.)), and by a Marie Skłodowska-Curie fellowship (E.J.R.) and FPI fellowships BES 2010-034552 (J.O.) and SVP-2013-067777 (S.V.). The cost of this publication has been paid in part with FEDER funds.
Author information
Authors and Affiliations
Contributions
M.R.C. and J.M.R conceived the study; J.O., N.M.-B., M.R.C. and J.M.R. designed the study and analyzed the data; J.O. and N.M.-B. performed most of the experiments, with contributions from E.J.R., S.V., L.I.C., R.A. and N.L.-V.; L.J.J.-B. supervised and analyzed the echography analysis; M.R., J.D.B., M.A.H. and J.F.N. provided human tissue samples; L.J.J.-B., M.R., A.M.B., M.A.H., D.M., A.E., M.S., J.F.N. and J.D.B. provided experimental support and ideas for the project; M.R.C. and J.M.R. wrote the manuscript with contributions from J.O. and N.M.-B. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
Supplementary Figures 1–9 (PDF 27413 kb)
Rights and permissions
About this article
Cite this article
Oller, J., Méndez-Barbero, N., Ruiz, E. et al. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med 23, 200–212 (2017). https://doi.org/10.1038/nm.4266
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4266
This article is cited by
-
In vivo phenotypic vascular dysfunction extends beyond the aorta in a mouse model for fibrillin-1 (Fbn1) mutation
Scientific Reports (2024)
-
Versican accumulation drives Nos2 induction and aortic disease in Marfan syndrome via Akt activation
EMBO Molecular Medicine (2024)
-
Molecular differences of angiogenic versus vessel co-opting colorectal cancer liver metastases at single-cell resolution
Molecular Cancer (2023)
-
The mechanism and therapy of aortic aneurysms
Signal Transduction and Targeted Therapy (2023)
-
The 10th International Conference on cGMP 2022: recent trends in cGMP research and development—meeting report
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)