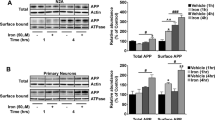
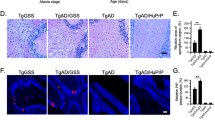
Abstract
The Alzheimer amyloid precursor protein (APP) is cleaved by several proteases, the most studied, but still unidentified ones, are those involved in the release of a fragment of APP, the amyloidogenic β-protein Aβ. Proteolysis by γ-secretase is the last processing step resulting in release of Aβ. Cleavage occurs after residue 40 of Aβ [Aβ(1–40)], occasionally after residue 42 [Aβ(1–42)]. Even slightly increased amounts of this Aβ(1–42) might be sufficient to cause Alzheimer's disease (AD) (reviewed in ref. 1, 2). It is thus generally believed that inhibition of this enzyme could aid in prevention of AD. Unexpectedly we have identified in neurons the endoplasmic reticulum (ER) as the site for generation of Aβ(1–42) and the trans-Golgi network (TGN) as the site for Aβ(1–40) generation. It is interesting that intracellular generation of Aβ seemed to be unique to neurons, because we found that nonneuronal cells produced significant amounts of Aβ(1–40) and Aβ(1–42) only at the cell surface. The specific production of the critical Aβ isoform in the ER of neurons links this compartment with the generation of Aβ and explains why primarily ER localized (mutant) proteins such as the presenilins3 could induce AD. We suggest that the earliest event taking place in AD might be the generation of Aβ(1–42) in the ER.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Younkin, S.G. Evidence that A beta 42 is the real culprit in Alzheimer's disease. Ann. Neural. 37, 287–288 (1995).
Hardy, J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosd. 20, 154–159 (1997).
Kovacs, D.M. et al. Alzheimer-associated presenilins 1 and 2: Neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nature Med. 2, 224–229 (1996).
Ida, N. et al. Analysis of heterogeneous bA4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive western blot assay. J. Biol. Chem. 271, 22908–22914 (1996).
Hartmann, T. et al. Alzheimer's disease beta A4 protein release and amyloid precursor protein sorting are regulated by alternative splicing. J. Biol. Chem. 271, 13208–13214 (1996).
De Strooper, B. et al. Production of intracellular amyloid-containing fragments in hippocampal neurons expressing human amyloid precursor protein and protection against amyloidogenesis by subtle amino acid substitutions in the rodent sequence. EMBO J. 14, 932–4938 (1995).
Simons, M. et al. Amyloidogenic processing of the human amyloid precursor protein in primary cultures of rat hippocampal neurons. J. Neurosd. 16, 899–908 (1996).
Tienari, P.J. et al. The β-amyloid domain is essential for axonal sorting of amyloid precursor protein. EMBO J. 15, 5218–5229 (1996).
Yamazaki, T., Selkoe, D.J. & Koo, E.H. Trafficking of cell surface beta-amyloid precursor protein: retrograde and transcytotic transport in cultured neurons. J. Cell Biol. 129, 431–442 (1995).
Haass, C. et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359, 322–325 (1992).
Haass, C. et al. Normal cellular processing of the beta-amyloid precursor protein results in the secretion of the amyloid beta peptide and related molecules. Ann. N. Y. Acad. Sci. 695, 109–116 (1993).
Shoji, M. et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258, 126–129 (1992).
Suzuki, N. et al. An increased percentage of long amyloid β protein secreted by familial amyloid b protein precursor (bAPP717) mutants. Science 264, 1336–1340 (1994).
Ida, N., Masters, C.L. & Beyreuther, K. Rapid cellular uptake of Alzheimer amyloid bA4 peptide by cultured human neuroblastoma cells. FEBS Lett. 394, 174–178 (1996).
Klafki, H.W., Paganetti, P.A., Sommer, B. & Staufenbiel, M. Calpain inhibitor I decreases beta A4 secretion from human embryonal kidney cells expressing beta-amyloid precursor protein carrying the APP670/671 double mutation. Neurosd. Lett. 201, 29–32 (1995).
Higaki, J., Quon, D., Zhong, Z. & Cordell, B. Inhibition of beta-amyloid formation identifies proteolytic precursors and subcellular site of catabolism. Neuron 14, 651–659 (1995).
Allsop, D. et al. Studies on inhibition of β-amyloid formation in APP-751-transfected IMR-32 cells, and SPA4CT transfected SHSY5Y cells. in Alzheimer's Disease: Biology, Diagnosis and Therapeutics (eds. Iqbal, K., Winblad, B., Nishimura, T., Takeda, M. & Wisiniewski, H.M.) 717–727 (Wiley & Sons, Chichester, 1997).
Fuller, S.J. et al. Intracellular production of beta A4 amyloid of Alzheimer's disease: Modulation by phosphoramidon and lack of coupling to the secretion ofthe amyloid precursor protein. Biochemistry 34, 8091–8098 (1995).
Tienari, P.J. et al. Intracellular and secreted Alzheimer's beta-amyloid species are generated by distinct mechanisms in cultured hippocampal neurons. Proc. Nat. Acad. Sci. USA 94, 4125–4130 (1997).
Turner, R.S., Suzuki, N., Chyung, A.S.C., Younkin, S.G. & Lee, V.M.Y. Amyloids beta(40) and beta(42) are generated intracellularly in cultured human neurons and their secretion increases with maturation. J. Biol. Chem. 271, 8966–8970 (1996).
Wild-Bode, C. et al. Intracellular generation and accumulation of amyloid β-peptide terminating at amino acid 42. J. Biol. Chem. 272, 16085–16088 (1997).
Scheuner, D. et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2and APP mutations linked to familial Alzheimer's disease. Nature Med. 2, 864–870 (1996).
Dyrks, T. et al. Generation of beta A4 from the amyloid protein precursor and fragments thereof. FEBS Lett. 335, 89–93 (1993).
Haass, C. et al. beta-Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J. Biol. Chem. 268, 3021–3024 (1993).
Citron, M. et al. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nature Med. 3, 67–72 (1997).
Gabuzda, D., Busciglio Chen, L.B., Matsudaira, P. & Yankner, 8.A. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J. Biol. Chem. 269, 13623–13628 (1994).
Jarrett, J.T., Berger, E.P. & Lansbury, P.T., Jr. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry 32, 4693–4697 (1993).
Bretscher, M.S. & Munro, S. Cholesterol and the Golgi apparatus. Science 261, 1280–1281 (1993).
Olkkonen, V.M., Liljestrom, P., Garoff, H., Simons, K. & Dotti, C.G. Expression of heterologous proteins in cultured rat hippocampal neurons using the Semliki Forest virus vector. J. Neurosci. Res 35, 445–451 (1993).
Mönning, U. et al. Extracellular matrix influences the biogenesis of amyloid precursor protein in microglial cells. J. Biol. Chem. 270, 7104–7110 (1995).
Hayat, M. ed. Fixation for Electron Microscopy. (Academic Press, New York, 1981).
Danscher, G. Localization of gold in biological tissue: A photochemical method for light and electron microscopy. Histochemistry 71, 81–88 (1981).
van de Plas, P. & Leunissen, J.L. Ultrasmall gold probes: Characteristics and use in immuno(cyto)chemical studies. Methods Cell Biol. 37, 241–257 (1993).
Heym, C. & Forssmann, W.-G. eds. Techniques in Neuroanatomical Research (Springer-Verlag, Heidelberg, 1981).
Strum, J.M., Wicken, J., Stanbury, J.R. & Karnovsky, M.J. Appearance and function of endogenous peroxidase in fetal rat thyroid. J.Cell Biol. 51, 162–175 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hartmann, T., Bieger, S., Brühl, B. et al. Distinct sites of intracellular production for Alzheimer's disease Aβ40/42 amyloid peptides. Nat Med 3, 1016–1020 (1997). https://doi.org/10.1038/nm0997-1016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0997-1016
This article is cited by
-
Amyloid fibril proteomics of AD brains reveals modifiers of aggregation and toxicity
Molecular Neurodegeneration (2023)
-
The interwoven fibril-like structure of amyloid-beta plaques in mouse brain tissue visualized using super-resolution STED microscopy
Cell & Bioscience (2023)
-
Current understandings and perspectives of petroleum hydrocarbons in Alzheimer’s disease and Parkinson’s disease: a global concern
Environmental Science and Pollution Research (2022)
-
Specific Mutations in the Cholesterol-Binding Site of APP Alter Its Processing and Favor the Production of Shorter, Less Toxic Aβ Peptides
Molecular Neurobiology (2022)
-
Sleep duration and efficiency are associated with plasma amyloid-β in non-demented older people
Neurological Sciences (2022)