Abstract
Recently, several genome-wide association studies (GWAS) on bipolar disorder (BPD) suggested novel risk genes. However, only few of them were followed up and further, the specificity of these genes is even more elusive. To address these issues, we genotyped SNPs in ANK3, CACNA1C, CMTM8, DGKH, EGFR, and NPAS3, which were significantly associated with BPD in previous GWAS, in a sample of 380 BPD patients. Replicated SNPs were then followed up in patients suffering from unipolar depression (UPD; n=387) or adult attention-deficit/hyperactivity disorder (aADHD; n=535). While we could not confirm an association of ANK3, CACNA1C, and EGFR with BPD, 10 SNPs in DGKH, CMTM8, and NPAS3 were nominally associated with disease, with two DGKH markers surviving correction for multiple testing. When these were followed up in UPD and aADHD, seven DGKH SNPs were also associated with UPD, while one SNP each in NPAS3 and CMTM8 and four in DGKH were linked to aADHD. Furthermore, a DGKH haplotype consisting of rs994856/rs9525580/rs9525584 GAT was associated with all disorders tested, while the complementary AGC haplotype was protective. The corresponding haploblock spans a 27-kb region covering exons coding for amino acids 65–243, and thus might include functional variants yet to be identified. We demonstrate an association of DGKH with BPD, UPD, and aADHD by applying a two-stage design. These disorders share the feature of mood instability, so that this phenotype might be associated with genetic variation in DGKH.
Similar content being viewed by others
INTRODUCTION
Bipolar disorder (BPD) is a severe psychiatric disorder and affects up to 4% of the adult population worldwide (Bauer and Pfennig, 2005; Merikangas et al, 2007). Approximately 20% of the patients die of suicide (Kilbane et al, 2009). Additionally, co-morbid disorders are frequent and include adult attention-deficit/hyperactivity disorder (aADHD), anxiety disorders, and substance abuse (Kessler et al, 2006; Merikangas et al, 2007). Taken together, this results in severe psychosocial adversity and leads to serious economic burden. The etiology of BPD remains largely unknown but it is evident that genetic factors have an important role (Kieseppa et al, 2004; McGuffin et al, 2003).
Consistently replicated risk genes for BPD are still lacking. However, a variety of research tools were applied to detect susceptibility genes for BPD. This includes linkage studies, candidate gene association studies, and finally genome-wide association studies (GWAS). More than 40 linkage scans for BPD have been published to date. Different meta-analysis found the strongest evidence for susceptibility loci on 13q and 22q (Segurado et al, 2003). In a combined analysis, 6q21–q25 and 8q24 showed genome-wide significance (McQueen et al, 2005). The underlying genes, however, have not yet been identified. With regard to candidate gene studies, several genes were shown to be associated with BPD, but none of them has been established as a specific BPD susceptibility gene. Among the best-replicated genes are DISC1, DAOA/G72, BDNF, TPH2, NRG1, ARNTL/CLOCK, and FAT (Barnett and Smoller, 2009). Another approach to identify genetic factors predisposing to diseases is the search for gross structural variations (Zhang et al, 2009).
As there are no common loci of large effect, but several genes with small effect sizes increasing the risk toward BPD, GWAS might be more fruitful. Using this rationale, several novel risk genes have recently been published. By examining an US and German population, Baum et al (2008a) reported genome-wide significance of rs10120253 in intron 1 of DGKH (diacylglycerol kinase eta). DGKH also is a promising functional candidate gene as its gene product is involved in the phosphatidyl inositol pathway, which is assumed to have an important role in lithium action. The UK Wellcome Trust Case–Control Consortium (WTCCC; WTCCC, 2007) demonstrated genome-wide significance for a marker next to PALB2, NDUFAB1, and DCTN5, and Sklar et al (2008) reported significant findings for MYO5B, TSPAN8, and EGFR. Finally, also including meta-analytic treatment of the WTCCC and Sklar data sets found strong evidence for CACNA1C (α-1 subunit of a voltage-dependent calcium channel) and ANK3 (ankyrin 3) (Ferreira et al, 2008). Taken together, these GWAS have provided risk genes that have been replicated in some cases (DGKH (Baum et al, 2008b; Ollila et al, 2009); SORCS2 and DFNB31 (Ollila et al, 2009); CACNA1C (Green et al, 2009); ANK3 (Lee et al, 2010; Schulze et al, 2009; Scott et al, 2009; Smith et al, 2009); TSPAN8 (Scholz et al, 2010)), while other replication attempts were negative. Several promising hits, however, were never attempted to replicate, and the potential impact of these candidate genes on other disorders displaying by mood disturbance has not yet been assessed. We have, therefore, picked the most promising risk genes and attempted their replication in an independent BPD sample. Confirmed risk genes thereafter were tested in samples consisting of patients suffering from unipolar depression (UPD) or aADHD, which is also characterized by severe mood dysregulation (Jacob et al, 2007).
MATERIALS AND METHODS
Samples
A detailed description of the BPD sample is available elsewhere (Lundorf et al, 2005; Reif et al, 2006b; Scholz et al, 2010). This sample consisted of 214 unrelated bipolar patients (mean age 51.7±14.1 years, 65% female), from the German Lower Franconia area for whom an ICD-10 diagnosis was established by means of an extensive, semi-structured interview analogous to the AMDP interview (AMDP, 2000) carried out by two experienced psychiatrists at the University of Würzburg. Furthermore, the OPCRIT system was used in these patients (McGuffin et al, 1991). A further 166 unrelated bipolar patients (mean age 43.0±11.5 years, 49% female) were ascertained according to ICD-10 diagnostic criteria for research (DCR) by means of a semi-structured interview (SCAN ver. 2.1.; World Health Organization, 1998) at the Center for Psychiatric Research, Århus University Hospital, giving a total number of 380 patients suffering from BPD. In all, 387 unrelated patients of German origin suffered from UPD and were enrolled also in the lower Franconia region (n=120, mean age 54.1±16.3 years, 54% female) as well as at the Department of Psychiatry, University of Münster (n=267, mean age 49.7±15.4 years, 57% female) as described (Baune et al, 2008). The diagnosis for UPD was ascertained by trained psychiatrists according to the ICD-10 DCR on the basis of semi-structured interviews. Co-morbidity data for UPD and BPD, respectively, with aADHD could not be obtained as these patients were ascertained during acute disease episodes where it is not possible to reliably establish a diagnosis of aADHD.
The aADHD study sample has been described previously (Franke et al, 2010a; Jacob et al, 2007; Gross-Lesch et al, in preparation) and consisted of 535 unrelated patients (mean age 33.7±10.2 years, 46% female), recruited at the University of Würzburg in the Lower Franconian region, who completed a structured interview from which diagnoses of DSM-IV aADHD were determined by two experienced psychiatrists. In all, 60% of the aADHD patients suffered from combined type ADHD, 30 of inattentive type ADHD, and 10% of hyperactive-impulsive type ADHD. In all, 57% of patients suffered from co-morbid lifetime depression, 32% of lifetime anxiety disorders, and 43% of lifetime substance abuse disorders (mainly alcohol abuse). BPD has been an exclusion criterion for these patients. None of the patients showed significant neurologic co-morbidity, mental retardation, or other somatic disorders, suggesting organic psychosis. Patients with substance-induced disorders were excluded as well.
The control sample consisted of 630 healthy subjects and was composed of blood donors, staff members, and volunteers all originating from the Lower Franconia region. A total of 284 control subjects (mean age 35±13 years, 47% female), consisting of healthy blood donors originating from Würzburg, were enrolled. The sample was not screened for psychiatric disorders; however, all subjects were free of medication, and the study was explained to them, so that the likelihood of severe psychiatric disorders in the control sample was low. An additional 356 subjects (mean age 33.7±10.2 years, 51% female) were recruited and screened for the absence of psychiatric disorders by conducting the Structured Clinical Interview for DSM-IV (SCID-I). All case as well as control subjects were of self-reported German, or Danish, respectively, ethnicity. Only subjects who gave written informed consent were enrolled in the study, which complied with the Declaration of Helsinki and was approved by the Ethics Committees of the Universities of Würzburg, Münster, and Århus.
SNP Selection and Genotyping
Genes of interest were compiled from published GWAS on BPD: NPAS3, ARNT2, CACNA1C, ANK3 (Ferreira et al, 2008), NXN, SLC39A3, SORCS2, DGKH (Baum et al, 2008a; Ollila et al, 2009), NALCN, SLC19A3, SLC29A3, DFNB31, CMTM8 (Ollila et al, 2009; WTCCC, 2007), and EGFR (Sklar et al, 2008). Genes were selected due to strength of association signals, biological rationale, and involved pathways; significant SNPs were selected from previous studies. The study focused on CACNA1C, ANK3, DGKH, CMTM8, and EGFR, as in our hands those were the most promising candidate genes. For the other genes, only one or two top SNPs were tested as pilot investigations; nevertheless, they were also included in this analysis to fully account for multiple testing. Taken together, 14 genes that contained associated SNPs were tagged with 99 SNPs. In a first step, we tested for an association of these SNPs with BPD (single marker data, Supplementary Table 1; haplotype analysis, Supplementary Table 2). Second, we genotyped the 23 SNPs that were found to be nominally associated with BPD (Table 1) in the UPD (single marker data, Supplementary Table 3; haplotype analysis, Supplementary Table 4) and aADHD (single marker data, Supplementary Table 5; haplotype analysis, Supplementary Table 6) samples to assess whether these SNP associations were specific for BPD.
SNP genotyping was performed using Sequenom's MassArray system (Sequenom, San Diego, CA) according to the instructions supplied by the manufacturer. All PCR reactions were done using the iPlex chemistry following the manufacturer's standard operation procedure. All primer sequences are given in Supplementary Table 7.
Statistical Analysis
Statistical analysis of genotype data was performed with PLINK V1.07. (Purcell et al, 2007) and HaploView V4.1 (Barrett et al, 2005). Only polymorphic SNPs with a call rate (CR) ⩾75% were included in the study; of those, genotype frequencies were ascertained for overall Hardy–Weinberg equilibrium (HWE; χ2 HWE p-value ⩾0.01). In the BPD sample, 87 of 99 typed SNPs passed these inclusion criteria, 12 SNPs accordingly were excluded from further analysis (rs41274688, rs35776153, rs999940, and 35065420 were monomorphic; rs4955274, rs11914777, rs17172438, and 7984523 departed from HWE; rs4411993, rs7683874, rs10234806, and 10994336 had a CR <75%). Of the 23 SNPs genotyped in UPD patients, three SNPs (rs4979416 and rs12496256 due to low CR and rs17455703 due to HWE departure) in the Würzburg subsample and one SNP (rs11773818, low CR) in the Münster subsample did not fulfill the inclusion criteria. In the aADHD sample, all 23 typed markers complied with the quality criteria.
Single marker associations were calculated by comparison of allele counts in 1-degree-of-freedom χ2 tests; results were adjusted for multiple testing using the conservative Bonferroni correction. For multi-marker association tests, haplotype blocks were defined according to the four-gamete rule (Wang et al, 2002); inferred haplotype counts in groups were compared with logistic regression. For each haplotype, this multi-marker association test was permuted 10 000 times to generate an empirical probability distribution; this was used to estimate p-values that control the family-wise error rate (FWER). With our study population, nominal association tests have a power of 55 and 50% to detect SNPs and haplotypes, respectively, conveying an odds ratio (OR) of 1.5 (corresponding to a relative risk of 1.48) to develop BPD assuming a co-dominant model and an MAF of 0.05 (Menashe et al, 2008). Using the same parameters, the power for SNP and haplotype associations was 66 and 64% for aADHD, while for UPD, the power is 59 and 56%, respectively.
Furthermore, meta-analytic treatment of rs9315885 and rs1170191 was performed by including the studies by Baum et al (2008a), Ollila et al (2009), and Squassina et al (2009) (as these SNPs were not genotyped in the study by Tesli et al (2009), this study could not be included in the meta-analysis): ORs were calculated as a measure for effect size; thereafter, the Q-statistic was applied to assess heterogeneity. Inconsistency across studies was quantified with I^2 metric (I^2=Q–df/Q). In the absence of heterogeneity, ORs were combined using fixed-effects models; if significant heterogeneity was detected, joint ORs were derived from random-effects models. Calculations were performed using R version 2.10 along with the package metafor version 0.5–7.
RESULTS
Single Marker Analysis
In order to replicate and assess the specificity of 14 selected genes from published GWAS on BPD, we analyzed 88 tag SNPs in the genes ANK3, ARNT2, CACNA1C, CMTM8, DFNB31, DGKH, EGFR, NALCN, NPAS3, NXN, SLC19A3, SLC29A3, SLC39A3, and SORCS2 in our BPD sample. After correction for multiple testing, two SNPs (rs1170169 and rs9525580) in DGKH remained significantly associated with BPD; at the nominal level, eight further significant findings were detected in the combined BPD sample, while another 13 SNPs were associated in only one of the BPD subsamples (Table 1; Supplementary Table 1). In order to examine the specificity of these associations for BPD, we further analyzed all 23 nominally associated markers (from the genes CMTM8, EGFR, DFNB31, DGKH, NPAS3, and SLC39A3) in UPD (Table 1; Supplementary Table 3) and aADHD (Table 1; Supplementary Table 5). SNPs from the other eight genes showed no significant association with BPD and were thus not analyzed further.
Cross-disorder genotyping revealed a total of 12 SNPs in four genes (CMTM8, DGKH, NPAS3, and SLC39A3) that were associated with at least one of the three examined phenotypes at the nominal level (Table 1). Ten association p-values were nominally significant in the combined BPD sample (the remaining other two had a borderline significant p=0.056), seven in the combined UPD sample, and six in the aADHD sample, with seven SNPs being associated with two and two SNPs with all three disorders. Only those SNPs that were associated with BPD in the combined sample replicated in either UPD or aADHD (however, including rs2148004 and rs347405 with p=0.056), but not those that were only associated in one of the subsamples (compare Supplementary Table 1 with Table 1). Most of the replicated SNPs mapped to DGKH (BPD: six SNPs; UPD: seven SNPs; aADHD: four SNPs), which was the only gene in our study that contained SNPs, which were significant following Bonferroni correction (Table 1). Noteworthy, all associations found with UPD mapped to DGKH. In CMTM8, only one SNP (rs6803740) featured an overlapping association between BPD and aADHD. The same disorders also overlapped in their association regarding the NPAS3 SNP rs7455703. The SLC39A3 association of rs4806874 was found to be exclusive for BPD (Table 1).
Haplotype Analysis
Haplotype analysis was then performed with all cross-disorder genotyped SNPs (BPD, significant findings: Table 2, complete data are given in Supplementary Table 2; UPD significant findings: Table 2, complete data are given in Supplementary Table 4; aADHD significant findings: Table 2, complete data are given in Supplementary Table 6). The strongest association found in all analyzed disorders was in DGKH block 2 (rs994856–rs9525580–rs9525584; Figure 1) haplotype GAT, which is exclusively composed of each single marker's risk alleles; this was consistent in all three examined phenotypes. Accordingly, GAT frequency was increased in all case groups as compared with controls; although this was nominally significant in all three disorders, the FWER was below 5% only in BPD and aADHD, but slightly above this threshold in UPD (permutation p=0.056). The GAT haplotype can, therefore, be assumed to predispose to at least two, but possibly to any of the three disorders (see Table 2). In terms of frequency, GAT follows its ‘complementary’ haplotype AGC, which is composed of those alleles that have a higher MAF in controls. The expected protective effect conveyed by AGC, however, was only significant in UPD following FWER correction, and nominally also in BPD (Table 2). A similar phenomenon was seen in DGKH block 1 (rs1170191–1170169–rs2148004) haplotype GCG, whose frequency was lower in all case groups as compared with controls, but the presumed protective effect was nominally significant only in BPD and UPD. The haplotype GGA in turn was enriched in all cases; following FWER, this was significant in BPD, whereas nominally it was also associated with aADHD and UPD, respectively (p=0.051; Table 2). Two further risk haplotypes were exclusively found to be associated with UPD (Table 2).
LD plot of DGKH, according to the four-gamete rule.
Haplotype associations in genes other than DGKH were found to be restricted to specific psychiatric disorders and did not withstand correction for multiple testing. In CMTM8, block 1 (rs6550109–rs12496256; Figure 2) haplotype TG was significantly protective, while block 3 (rs4276227–rs6803740) haplotype CG was associated with risk for BPD (Table 2). The CMTM8 block 2 (rs4955272–rs7644602–rs7632109) haplotype GGG was the only significant haplotype association in aADHD and presumed to be protective (Table 2). NPAS3 rs8015959–rs17455703 had two alleles associated with BPD, the protective CG and the risk haplotype CA (Table 2).
LD plot of CMTM8, according to the four-gamete rule.
Meta-Analysis
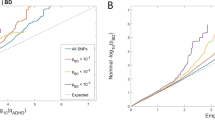
To compare our findings with previous studies, we have subjected the significant SNPs rs9315885 and rs1170191 from the studies by Baum et al (2008a), Ollila et al (2009), and Squassina et al (2009) to a formal meta-analysis (Figure 3; Supplementary Table 8). While rs9315885, which was significant in the three other studies but not our data set, proved to be highly significantly associated in the meta-analysis (Figure 3a), this was not the case for rs1170191, as the effect direction was reversed in our as compared with other studies (Figure 3b).
Forest plots displaying meta-analyses of minor vs major allele of rs9315885 (a) and rs1170191 (b).
DISCUSSION
The present study had two major aims: (1) to replicate risk genes for BPD obtained through GWAS and (2) to assess the specificity of associated risk genes by testing all nominally associated SNPs in UPD and aADHD as well. We chose to specifically test risk variants in CACNA1C, ANK3, DGKH, CMTM8, and EGFR, while for several other genes only a few SNPs were tested, which we report here as well to fully account for multiple testing. In the following discussion, we will elaborate on a gene-by-gene wise manner, yet genes where only a few SNPs were tested and not found to be associated will not be commented further upon (ARNT2, DFNB31, NALCN, NXN, SLC19A3, SLC29A3, SLC39A3, and SORCS2). An important caveat that has to be considered in the interpretation of our data is the use of a single control group, which has been compared against all three diagnostic groups. Significant deviation of our control group from the population's allele distribution, therefore, would bias our association data. We have thus compared the MAFs of our control sample to a set of German population based controls, which have been ascertained via the HNR/KORA studies and genotyped on an Illumina microarray (n=795; M Mattheisen and S Cichon, personal communication). From the 86 SNPs entering our analysis, 52 could be retrieved from the microarray. Only one of these 52 SNPs however significantly differed from our control sample (nominal p-value p=0.017, rs1370717; all other p-values were >0.15, data not shown), which argues against the assumption of a systematic bias due to the use of an unusual control group.
ANK3 and CACNA1C
In our panel of candidate genes, we have also included the top SNPs of the most replicated BPD risk genes so far: ANK3 (Ferreira et al, 2008; Lee et al, 2010; Schulze et al, 2009; Scott et al, 2009; Smith et al, 2009), which was also associated with schizophrenia (Athanasiu et al, 2010) and CACNA1C (Ferreira et al, 2008; Keers et al, 2009; Sklar et al, 2008; WTCCC, 2007), which was as well demonstrated to be associated with schizophrenia (Green et al, 2009; Moskvina et al, 2009; Nyegaard et al, 2010), UPD (Green et al, 2009), and its endophenotypes (Casamassima et al, 2010). CACNA1C was shown to exert effects on verbal fluency and functional (Erk et al, 2010; Krug et al, 2010; Wessa et al, 2010) and structural (Franke et al, 2010b; Kempton et al, 2009) neuroimaging. We have aimed to replicate these genes in our bipolar sample, yet there was no significant association of either ANK3 or CACNAC1C so that we abstained from testing them further. Several reasons for this lack of replication have to be considered: (1) lack of power owing to the sample size of n=380 bipolar patients, as compared with the huge number of patients tested in current GWAS; (2) missed common variants, as we did not tag the whole gene but rather focused on previously associated SNPs however, including CACNA1C rs1006737, which was tested in the genomic imaging studies outlined above; (3) missed rare variants causing an association of common variants in the discovery samples (Dickson et al, 2010), thus escaping replication attempts due to differing LD substructures in the examined population; and (4) genetic heterogeneity of BPD, resulting in an association of risk genes in some, but not all populations (which might be very likely as associations were hitherto restricted to US American, UK, and Irish populations in the case of CACNA1C).
CMTM8 and EGFR
These two genes are considered together, as CMTM8 (CKLF-like MARVEL transmembrane domain containing 8) appears to be a negative regulator of EGF-induced signaling (Jin et al, 2005, 2007), which is mediated by the EGF receptor EGFR (previously termed ErbB). Thus, a common pathway of EGFR and CMTM8 seems reasonable. While the first evidence for an involvement of EGFR in BPD came from the GWAS by Sklar et al (2008), CMTM8 was identified in the WTCCC data set (WTCCC, 2007) yet not replicated later (Ollila et al, 2009). While there is almost no information on CMTM8, there is a vast body of literature on EGFR. This receptor kinase signals through PI3K/Akt as well as RAS/RAF/MEK/ERK (Wong and Guillaud, 2004), leading to downstream mechanisms including cell proliferation and survival. Accordingly, EGFR was shown to regulate neural stem cell proliferation (Cesetti et al, 2009; Grimm et al, 2009; Suh et al, 2009) and migration (Kim et al, 2009). Most interestingly, NO exerts its effect on neural stem cell proliferation by preventing EGFR-induced Akt phosphorylation (Torroglosa et al, 2007). Thus, EGFR and it regulators are excellent candidate molecules for neuropsychiatric disorders. Although other ErbB isoforms have gained much interest due to their interaction with neuregulin-1 (Birchmeier, 2009), there are almost no studies on EGFR/ErbB1 and its pathway. In the present study, we could however not find support for an involvement of EGFR variation in BPD, in contrast to Sklar et al (2008). Again, this might be due to population-specific associations, yet the independent finding on CMTM8 in the WTCCC GWAS underscores the notion that GWAS, when combined, can indentify novel pathways and thereby provide a starting point for more mechanistic studies. As we could replicate CMTM8 as a bipolar—and, with borderline significance, aADHD—risk gene, we consider further studies worthwhile. Interestingly, when looking up CMTM8 in our pooled GWAS on aADHD (Lesch et al, 2008), rs9833771 which is just 18 kb away from our most significant CMTM8 finding rs6803740 was associated with aADHD at p=0.0002, adding further support for our notion that it is involved in aADHD.
NPAS3
Several lines of evidence link the transcription factor neuronal PAS domain protein 3 (NPAS3) to psychiatric disorders (Pickard et al, 2006). Kamnasaran et al (2003) reported on a family in which a disruption of NPAS3 segregates with schizophrenia and was also associated with learning disability (Pickard et al, 2005). This finding was picked up soon thereafter in animal studies, demonstrating that Npas3 deletion mutant mice display schizophrenia-like behavioral abnormalities (Erbel-Sieler et al, 2004). Most interestingly, this was paralleled by a marked reduction of hippocampal adult neurogenesis (Pieper et al, 2005), which is suggested to have a role in schizophrenia (Reif et al, 2006a). Later, Pickard et al (2009) could show that common genetic variation in the NPAS3 gene is associated with both schizophrenia and BPD. Furthermore, coding non-synonymous variants were identified and demonstrated to be associated with schizophrenia (Macintyre et al, 2010), which might well underlie the association of common, intronic variants (Dickson et al, 2010). Additionally, NPAS3 was identified in two GWAS to be associated with iloperidone response (Lavedan et al, 2009) and, interestingly, BPD (Ferreira et al, 2008). Thus, both hypothesis-free and hypothesis-driven genetic data as well as animal models argue for a role of NPAS3 in psychoses. Indeed, one (rs17455703) of the two NPAS3 SNPs tested in the present study again was associated with BPD. A nominally significant association for the same SNP with aADHD was also found, arguing that the connection between NPAS3 and psychiatric disorders crosses diagnostic boundaries. In line with this, three out of 282 NPAS3 SNPs tested in our aADHD GWAS (Lesch et al, 2008) were associated with disease, also following correction for multiple testing on a gene-based level (rs4503707, rs10483437, rs12100538). Further studies have to reveal whether NPAS3 is involved in cognitive functioning or rather emotional regulation, as both domains are affected in all three disorders (schizophrenia, BPD, and aADHD).
DGKH
The most prominent finding of our study however related to DGKH, suggested to be associated with BPD in the GWAS by Baum et al (2008a, 2008b). However, replication failed in studies on BPD and lithium response (Manchia et al, 2009; Tesli et al, 2009), while two other studies were ambiguous (Ollila et al, 2009; Squassina et al, 2009). We attempted to replicate previously associated SNPs (rs9315885 and rs1170191), and by calculating a meta-analysis a role for rs9315885 was confirmed (Figure 3a), which however was not due to a signal in our sample. Our most significant SNPs were tagging SNPs, which have not been previously reported. While our data thus are again arguing for a role of DGKH in BPD, they cannot be considered a replication in a strict statistical sense and thus follow-up studies have to further test the top SNPs described here. Nevertheless, a recent report demonstrated increased expression of DGKH in BPD (Moya et al, 2010), lending further support to the notion for an involvement of this molecule in BPD. The role of DGKH is to metabolize diacylglycerol (DAG), which is produced upon cleavage of PIP2 into IP3 and DAG by phospholipase C. DAG, in turn, activates protein kinase C (PKC), which phosphorylates a variety of proteins including Disheveled, an inhibitor of GSK3β. Thus, although the precise role of DGKH is not known yet, it clearly is involved in crucial pathways for psychiatric disorders and especially the mechanism of action of lithium. Intriguingly, DGKH knockdown in HeLa cells impaired the MEK/ERK pathway activated by EGF, while overexpression of the gene activated the pathway (Yasuda et al, 2009). Thus, DGKH is also linked to EGFR/CMTM8 mentioned above.
In our study, 6 out of 21 tested SNPs in DGKH were associated with BPD, and two SNPs withstood correction for multiple testing. Re-analysis of our aADHD GWAS (Lesch et al, 2008) revealed that 8 out of 52 SNPs were nominally associated with disease, one of which also survived correction on the gene level (rs10492444; nominal p=0.0004, corrected p=0.0212) and was located 505 bp away from rs9525584 being at the 3′ end of the risk haploblock delineated below. Furthermore, two frequent haplotypes were significantly associated with disease, especially rs994856/rs9525580/rs9525584 GAT. Apart from rs347405, all associated SNPs were also nominally associated with UPD (with three SNPs surviving correction for multiple testing) and again the haploblock rs994856/rs9525580/rs9525584 was associated. In aADHD, four of the eight SNPs replicated, one of which withstanding correction and again, rs994856/rs9525580/rs9525584 GAT was associated with disease. When looking at absolute haplotype frequencies, it becomes apparent that the GAT haplotype is always more frequent in cases (controls, 23%; BPD, 31%; UPD, 29%; aADHD, 30%), while the AGC haplotype is always less frequent in cases (controls, 48%; BPD, 42%; UPD, 39%; aADHD, 45%). While our data as well as HapMap CEU suggest that AGT are the major alleles, the sub-Saharan HapMap subset suggest that the AGC alleles are evolutionary older, that is the AGC haplotype can be considered ancient. The GAT haplotype appears to be evolutionary younger and thus it seems to convey the risk variant. The haploblock spans a genomic region of 27 kb covering exons two to six of the gene, that is amino acids 65–243. Three non-synonymous variants (no frequency data available) are deposited in databases, rs59790803 causing a G>A transition resulting in a possibly damaging Asp>Asn exchange (Polyphen-2 (Adzhubei et al, 2010) score: 0.736, sensitivity: 0.81, specificity: 0.90) in exon 6 at position 107; rs9566925, causing a stop mutation at position 90; and rs1344286, causing a A>C transition, resulting in a Thr>Pro exchange at position 65. These regions contain a pleckstrin homology (PH) domain (AA 65–158) and a phorbol-ester/DAG-type zinc finger (AA 175–225). While the latter is the DAG sensor, the PH binds phosphatidylinositols and proteins such as PKC. This domain is, therefore, involved in intracellular targeting and enables DGKH to interact with other signal transduction pathways. Both variants, therefore, might well alter the function of the protein by either impairing catalysis or changing protein–protein interactions, thereby disturbing intraneuronal second- and third-messenger pathways.
Outlook and Conclusions
By applying a two-stage design, we here demonstrated an association of DGKH with BPD, UPD, and aADHD. These disorders share the feature of mood instability with varying amplitude and frequency. Thus, genetic variation at the DGKH locus might be associated with this psychopathological phenotype. This is yet another example that a common genetic variant is associated with more than one psychiatric phenotype, which was also the case with other risk genes picked up by GWAS, for example, CACNA1C and ANK3. The integration of such findings might pinpoint distinct molecular pathways whose identification might enhance psychiatric diagnostics and research on neurobiological underpinnings.
References
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al (2010). A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249.
Arbeitsgemeinschaft, für Methodik und Dokumentation in der Psychiatrie (2000). Das AMDP-System. Manual zur Dokumentation Psychiatrischer Befunde. Hogrefe: Göttingen, Germany.
Athanasiu L, Mattingsdal M, Kahler AK, Brown A, Gustafsson O, Agartz I et al (2010). Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res 44: 748–753.
Barrett JC, Fry B, Maller J, Daly MJ (2005). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265.
Barnett JH, Smoller JW (2009). The genetics of bipolar disorder. Neuroscience 164: 331–343.
Bauer M, Pfennig A (2005). Epidemiology of bipolar disorders. Epilepsia 46 (Suppl 4): 8–13.
Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B et al (2008a). A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry 13: 197–207.
Baum AE, Hamshere M, Green E, Cichon S, Rietschel M, Noethen MM et al (2008b). Meta-analysis of two genome-wide association studies of bipolar disorder reveals important points of agreement. Mol Psychiatry 13: 466–467.
Baune BT, Hohoff C, Berger K, Neumann A, Mortensen S, Roehrs T et al (2008). Association of the COMT val158met variant with antidepressant treatment response in major depression. Neuropsychopharmacology 33: 924–932.
Birchmeier C (2009). ErbB receptors and the development of the nervous system. Exp Cell Res 315: 611–618.
Casamassima F, Huang J, Fava M, Sachs GS, Smoller JW, Cassano GB et al (2010). Phenotypic effects of a bipolar liability gene among individuals with major depressive disorder. Am J Med Genet B Neuropsychiatr Genet 153B: 303–309.
Cesetti T, Obernier K, Bengtson CP, Fila T, Mandl C, Holzl-Wenig G et al (2009). Analysis of stem cell lineage progression in the neonatal subventricular zone identifies EGFR+/NG2- cells as transit-amplifying precursors. Stem Cells 27: 1443–1454.
Dickson SP, Wang K, Krantz I, Hakonarson H, Goldstein DB (2010). Rare variants create synthetic genome-wide associations. PLoS Biol 8: e1000294.
Erbel-Sieler C, Dudley C, Zhou Y, Wu X, Estill SJ, Han T et al (2004). Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors. Proc Natl Acad Sci U S A 101: 13648–13653.
Erk S, Meyer-Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P et al (2010). Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch Gen Psychiatry 67: 803–811.
Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L et al (2008). Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 40: 1056–1058.
Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hummer A et al (2010a). Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology 35: 656–664.
Franke B, Vasquez AA, Veltman JA, Brunner HG, Rijpkema M, Fernandez G (2010b). Genetic Variation in CACNA1C, a Gene Associated with Bipolar Disorder, Influences Brainstem Rather than Gray Matter Volume in Healthy Individuals. Biol Psychiatry 68: 586–588 .
Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S et al (2009). The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry 15: 1016–1022.
Grimm I, Messemer N, Stanke M, Gachet C, Zimmermann H (2009). Coordinate pathways for nucleotide and EGF signaling in cultured adult neural progenitor cells. J Cell Sci 122 (Part 14): 2524–2533.
Jacob CP, Romanos J, Dempfle A, Heine M, Windemuth-Kieselbach C, Kruse A et al (2007). Co-morbidity of adult attention-deficit/hyperactivity disorder with focus on personality traits and related disorders in a tertiary referral center. Eur Arch Psychiatry Clin Neurosci 257: 309–317.
Jin C, Ding P, Wang Y, Ma D (2005). Regulation of EGF receptor signaling by the MARVEL domain-containing protein CKLFSF8. FEBS Lett 579: 6375–6382.
Jin C, Wang Y, Han W, Zhang Y, He Q, Li D et al (2007). CMTM8 induces caspase-dependent and -independent apoptosis through a mitochondria-mediated pathway. J Cell Physiol 211: 112–120.
Kamnasaran D, Muir WJ, Ferguson-Smith MA, Cox DW (2003). Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J Med Genet 40: 325–332.
Keers R, Farmer AE, Aitchison KJ (2009). Extracting a needle from a haystack: reanalysis of whole genome data reveals a readily translatable finding. Psychol Med 39: 1231–1235.
Kempton MJ, Ruberto G, Vassos E, Tatarelli R, Girardi P, Collier D et al (2009). Effects of the CACNA1C risk allele for bipolar disorder on cerebral gray matter volume in healthy individuals. Am J Psychiatry 166: 1413–1414.
Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RM et al (2006). Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am J Psychiatry 163: 1561–1568.
Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J (2004). High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry 161: 1814–1821.
Kilbane EJ, Gokbayrak NS, Galynker I, Cohen L, Tross S (2009). A review of panic and suicide in bipolar disorder: does comorbidity increase risk? J Affect Disord 115: 1–10.
Kim Y, Comte I, Szabo G, Hockberger P, Szele FG (2009). Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration. PLoS One 4: e8122.
Krug A, Nieratschker V, Markov V, Krach S, Jansen A, Zerres K et al (2010). Effect of CACNA1C rs1006737 on neural correlates of verbal fluency in healthy individuals. Neuroimage 49: 1831–1836.
Lavedan C, Licamele L, Volpi S, Hamilton J, Heaton C, Mack K et al (2009). Association of the NPAS3 gene and five other loci with response to the antipsychotic iloperidone identified in a whole genome association study. Mol Psychiatry 14: 804–819.
Lee MT, Chen CH, Lee CS, Chen CC, Chong MY, Ouyang WC et al (2010). Genome-wide association study of bipolar I disorder in the Han Chinese population. Mol Psychiatry.
Lesch KP, Timmesfeld N, Renner TJ, Halperin R, Roser C, Nguyen TT et al (2008). Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm 115: 1573–1585.
Lundorf MD, Buttenschon HN, Foldager L, Blackwood DH, Muir WJ, Murray V et al (2005). Mutational screening and association study of glutamate decarboxylase 1 as a candidate susceptibility gene for bipolar affective disorder and schizophrenia. Am J Med Genet B Neuropsychiatr Genet 135B: 94–101.
Macintyre G, Alford T, Xiong L, Rouleau GA, Tibbo PG, Cox DW (2010). Association of NPAS3 exonic variation with schizophrenia. Schizophr Res 120: 143–149.
Manchia M, Squassina A, Congiu D, Chillotti C, Ardau R, Severino G et al (2009). Interacting genes in lithium prophylaxis: preliminary results of an exploratory analysis on the role of DGKH and NR1D1 gene polymorphisms in 199 Sardinian bipolar patients. Neurosci Lett 467: 67–71.
McGuffin P, Farmer A, Harvey I (1991). A polydiagnostic application of operational criteria in studies of psychotic illness: development and reliability of the OPCRIT system. Arch Gen Psychiatry 48: 764–770.
McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A (2003). The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry 60: 497–502.
McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW et al (2005). Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet 77: 582–595.
Menashe I, Rosenberg PS, Chen BE (2008). PGA: power calculator for case-control genetic association analyses. BMC Genet 9: 36.
Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M et al (2007). Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64: 543–552.
Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E et al (2009). Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol Psychiatry 14: 252–260.
Moya PR, Murphy DL, McMahon FJ, Wendland JR (2010). Increased gene expression of diacylglycerol kinase eta in bipolar disorder. Int J Neuropsychopharmacol 13: 1–2.
Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, Sorensen KM et al (2010). CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry 15: 119–121.
Ollila HM, Soronen P, Silander K, Palo OM, Kieseppa T, Kaunisto MA et al (2009). Findings from bipolar disorder genome-wide association studies replicate in a Finnish bipolar family-cohort. Mol Psychiatry 14: 351–353.
Pickard BS, Christoforou A, Thomson PA, Fawkes A, Evans KL, Morris SW et al (2009). Interacting haplotypes at the NPAS3 locus alter risk of schizophrenia and bipolar disorder. Mol Psychiatry 14: 874–884.
Pickard BS, Malloy MP, Porteous DJ, Blackwood DH, Muir WJ (2005). Disruption of a brain transcription factor, NPAS3, is associated with schizophrenia and learning disability. Am J Med Genet B Neuropsychiatr Genet 136B: 26–32.
Pickard BS, Pieper AA, Porteous DJ, Blackwood DH, Muir WJ (2006). The NPAS3 gene—emerging evidence for a role in psychiatric illness. Ann Med 38: 439–448.
Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC et al (2005). The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci USA 102: 14052–14057.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575.
Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A et al (2006a). Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 11: 514–522.
Reif A, Herterich S, Strobel A, Ehlis AC, Saur D, Jacob CP et al (2006b). A neuronal nitric oxide synthase (NOS-I) haplotype associated with schizophrenia modifies prefrontal cortex function. Mol Psychiatry 11: 286–300.
Scholz CJ, Jacob CP, Buttenschon HN, Kittel-Schneider S, Boreatti-Hummer A, Zimmer M et al (2010). Functional variants of TSPAN8 are associated with bipolar disorder and schizophrenia. Am J Med Genet B Neuropsychiatr Genet 153B: 967–972.
Schulze TG, Detera-Wadleigh SD, Akula N, Gupta A, Kassem L, Steele J et al (2009). Two variants in ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol Psychiatry 14: 487–491.
Scott LJ, Muglia P, Kong XQ, Guan W, Flickinger M, Upmanyu R et al (2009). Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc Natl Acad Sci USA 106: 7501–7506.
Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger Jr JI et al (2003). Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: bipolar disorder. Am J Hum Genet 73: 49–62.
Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K et al (2008). Whole-genome association study of bipolar disorder. Mol Psychiatry 13: 558–569.
Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W et al (2009). Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry 14: 755–763.
Squassina A, Manchia M, Congiu D, Severino G, Chillotti C, Ardau R et al (2009). The diacylglycerol kinase eta gene and bipolar disorder: a replication study in a Sardinian sample. Mol Psychiatry 14: 350–351.
Suh Y, Obernier K, Holzl-Wenig G, Mandl C, Herrmann A, Worner K et al (2009). Interaction between DLX2 and EGFR regulates proliferation and neurogenesis of SVZ precursors. Mol Cell Neurosci 42: 308–314.
Tesli M, Kahler AK, Andreassen BK, Werge T, Mors O, Mellerup E et al (2009). No association between DGKH and bipolar disorder in a Scandinavian case-control sample. Psychiatr Genet 19: 269–272.
Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C, Matarredona ER, Campos-Caro A, Estrada C (2007). Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells 25: 88–97.
Wang N, Akey JM, Zhang K, Chakraborty R, Jin L (2002). Distribution of recombination crossovers and the origin of haplotype blocks: the interplay of population history, recombination, and mutation. Am J Hum Genet 71: 1227–1234.
Wessa M, Linke J, Witt SH, Nieratschker V, Esslinger C, Kirsch P et al (2010). The CACNA1C risk variant for bipolar disorder influences limbic activity. Mol Psychiatry 15: 1126–1127.
Wong RW, Guillaud L (2004). The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev 15: 147–156.
World Health Organization (1998). Diagnosis and clinical measurement in psychiatry. A reference manual for SCAN.
WTCCC (2007). Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447: 661–678.
Yasuda S, Kai M, Imai S, Takeishi K, Taketomi A, Toyota M et al (2009). Diacylglycerol kinase eta augments C-Raf activity and B-Raf/C-Raf heterodimerization. J Biol Chem 284: 29559–29570.
Zhang D, Cheng L, Qian Y, Alliey-Rodriguez N, Kelsoe JR, Greenwood T et al (2009). Singleton deletions throughout the genome increase risk of bipolar disorder. Mol Psychiatry 14: 376–380.
Acknowledgements
We are grateful to all patients for their participation in the study. This study was supported by the DFG (Grant RE1632/5-1 to AR, KFO 125 to AR and KPL; SFB 581 to KPL, SFB TRR 58 Z02 to JD and AR, C02 to KD, and A1/A5 to KPL), BMBF (IZKF N-27-N to AR), and the EC (NEWMOOD LSHM-CT-2003-503474 to KPL). We thank U Walter and M Zimmer for their kind help in operating the mass spectrometer. T Töpner, C Dreher, N Steigerwald, C Gagel, and J Auer are credited for excellent technical assistance. We thank M Mattheisen and S Cichon (Institute of Human Genetics, Department of Genomics, Life&Brain Center (IHG), University of Bonn) for providing us with the allele frequency data from the HNR/KORA studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AR and KD have received funding by Astra Zeneca. KD is on the speakers’ board of Pfizer, Lilly, and Bristol-Myers Squibb. These affiliations have no relevance to the work covered in the manuscript. None of the other authors reported any biomedical financial interests or potential conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Weber, H., Kittel-Schneider, S., Gessner, A. et al. Cross-Disorder Analysis of Bipolar Risk Genes: Further Evidence of DGKH as a Risk Gene for Bipolar Disorder, but also Unipolar Depression and Adult ADHD. Neuropsychopharmacol 36, 2076–2085 (2011). https://doi.org/10.1038/npp.2011.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.98
Keywords
This article is cited by
-
Molecular signature of excessive female aggression: study of stressed mice with genetic inactivation of neuronal serotonin synthesis
Journal of Neural Transmission (2023)
-
Identification of genetic overlap and novel risk loci for attention-deficit/hyperactivity disorder and bipolar disorder
Molecular Psychiatry (2021)
-
The genetics of bipolar disorder
Molecular Psychiatry (2020)
-
Reprogramming fatty acyl specificity of lipid kinases via C1 domain engineering
Nature Chemical Biology (2020)
-
Genetic and epigenetic analyses of panic disorder in the post-GWAS era
Journal of Neural Transmission (2020)