Abstract
Continued methamphetamine (MA) use is dependent on a positive MA experience and is likely attenuated by sensitivity to the aversive effects of MA. Bidirectional selective breeding of mice for high (MAHDR) or low (MALDR) voluntary consumption of MA demonstrates a genetic influence on MA intake. Quantitative trait locus (QTL) mapping identified a QTL on mouse chromosome 10 that accounts for greater than 50% of the genetically-determined differences in MA intake in the MAHDR and MALDR lines. The trace amine-associated receptor 1 gene (Taar1) is within the confidence interval of the QTL and encodes a receptor (TAAR1) that modulates monoamine neurotransmission and at which MA serves as an agonist. We demonstrate the existence of a non-functional allele of Taar1 in the DBA/2J mouse strain, one of the founder strains of the selected lines, and show that this non-functional allele co-segregates with high MA drinking and with reduced sensitivity to MA-induced conditioned taste aversion (CTA) and hypothermia. The functional Taar1 allele, derived from the other founder strain, C57BL/6J, segregates with low MA drinking and heightened sensitivity to MA-induced CTA and hypothermia. A role for TAAR1 in these phenotypes is corroborated in Taar1 transgenic mice: Taar1 knockout mice consume more MA and exhibit insensitivity to MA-induced CTA and hypothermia, compared with Taar1 wild-type mice. These are the first data to show that voluntary MA consumption is, in part, regulated by TAAR1 function. Behavioral and physiological studies indicate that TAAR1 function increases sensitivity to aversive effects of MA, and may thereby protect against MA use.
Similar content being viewed by others
INTRODUCTION
Functional brain circuitry flexibly encodes and responds to rewarding and aversive motivational states and events. Methamphetamine (MA) is highly addictive, but has both rewarding and aversive effects that influence use. Prevention and treatment rely on knowledge of the neural mechanisms that contribute to risk for addiction and to sensitivity to the motivational effects of MA. Replicated sets of mouse lines, bidirectionally selectively bred for high or low MA drinking (MADR), are of particular relevance to the study of genetic risk for human MA use. First, in this genetic model, MA intake is completely voluntary. Second, MA high drinking (MAHDR) mice show increased sensitivity to MA reinforcement in operant intracranial and oral self-administration procedures, whereas MA low drinking (MALDR) mice do not. Third, compared with MALDR mice, MAHDR mice have greater sensitivity to conditioned rewarding effects of MA that are relevant to relapse. Finally, MAHDR mice show little sensitivity to aversive effects of MA in conditioned place and conditioned taste aversion (CTA) assays, whereas MALDR mice exhibit high sensitivity. The genetically-determined, robust sensitivity to aversive effects in MALDR mice likely limits their MA intake (Shabani et al, 2011, 2012a, 2012b; Wheeler et al, 2009).
In addition to its well-known action as a substrate for neurotransmitter and vesicular monoamine transporters (Fleckenstein et al, 2007), MA is an agonist at trace amine-associated receptor 1 (TAAR1) (Bunzow et al, 2001; Wolinsky et al, 2007). Activation of TAAR1 appears to counteract some effects of MA. For example, pretreatment with the TAAR1 agonist, RO5263397, reduces operant self-administration of MA in rats (Jing et al, 2015). Trace amines, such as p-tyramine, β-phenylethylamine, octopamine, and tryptamine, interact with this G protein-coupled receptor (Borowsky et al, 2001; Bunzow et al, 2001; Lindemann et al, 2005; Wolinsky et al, 2007), and TAAR1 modulates monoamine activity, in part, through regulation of neurotransmitter availability and disposition (Revel et al, 2011; Xie and Miller, 2008). TAAR1 agonists reduce endogenous firing of dopaminergic (DA), noradrenergic (NE), and serotonergic (5-HT) neurons, and Taar1 knockout (−/−) mice exhibit greater amphetamine-induced release of these neurotransmitters in the striatum, compared with wild-type (+/+) littermates (Lindemann et al, 2008; Wolinsky et al, 2007). Taar1 −/− mice also display greater locomotor stimulation to amphetamine and MA (Achat-Mendes et al, 2012; Lindemann et al, 2008; Wolinsky et al, 2007), consistent with the idea that TAAR1 function is important for counteracting some MA effects. However, the role of TAAR1 function in sensitivity to aversive effects of MA has not been examined.
Physiological effects of MA contribute to subjective effects that could impact MA consumption. Acute and chronic MA can induce hyperthermia (Bowyer et al, 1994; Sabol et al, 2013), but hypothermia may occur at lower doses and 18–20 ° C ambient temperatures (Sabol et al, 2013). MA-induced hypothermia may be partly regulated by TAAR1, since other TAAR1 agonists induce hypothermia (Di Cara et al, 2011; Fantegrossi et al, 2013; Panas et al, 2010), and Taar1−/− mice do not exhibit hypothermia to doses of the MA-like drug, MDMA, that induce hypothermia in +/+ mice (Di Cara et al, 2011). Likewise, Taar1 −/− mice do not display hypothermia to MA, but the dose of MA used in this published study did not induce hypothermia in +/+ mice (Panas et al, 2010), so further study is needed.
Taar1 is within the confidence interval for a quantitative trait locus (QTL) on mouse chromosome 10 (Figure 1) that accounts for greater than 50% of the genetic variance in MA intake in MADR mice (Belknap et al, 2013). DBA/2J (D2) mice possess a unique non-synonymous single-nucleotide polymorphism (SNP, C229A) in Taar1 (Sanger Mouse Genomes Project SNP browser, 2014; Keane et al, 2011; Yalcin et al, 2011) that is not present in C57BL/6J (B6) mice. These are the two progenitor strains of the MADR lines. This polymorphism supports investigation of Taar1 as a viable candidate for a quantitative trait gene (QTG) regulating MA intake.
A quantitative trait locus (QTL) on mouse chromosome 10 has a major effect on MA drinking in MADR mice. The QTL on proximal chromosome 10 (10–40 Mb) explains greater than 50% of the genetic variance in the MA drinking phenotype (Belknap et al, 2013). Data are presented as directional genome-wide logarithm of the odds (LOD) scores. Positive and negative LOD scores indicate that higher trait scores are conferred by the D2 and B6 alleles, respectively. Dashed horizontal lines indicate statistically significant support for a QTL at p<2 × 10−5. Although there are a large number of genes in this interval that have the potential to influence the MA drinking trait, of particular interest based on the literature supporting involvement of opioid, glutamate, and TAAR1 in MA responses, are the mu opioid receptor gene (Oprm1), metabotropic glutamate receptor gene (mGlur1), and Taar1, which reside at 6.76, 10.7, and 23.9 Mb, respectively. Data shown were generated using two independent sets of replicated MADR lines, produced 2 years apart. Also shown are combined data for the independent replications of the QTL study. Figure adapted from Belknap et al (2013), with permission.
Here, we present frequency data in MADR mice for the Taar1 polymorphism found in D2 and B6 mice. We test the hypothesis that voluntary MA consumption is influenced by Taar1 by measuring MA intake in Taar1 −/−, heterozygous (+/−) and +/+ littermates. We also test the hypothesis that Taar1 has a role in sensitivity to a conditioned aversive effect of MA and in the thermal response to MA. In addition, we examine thermal response to ethanol to determine drug specificity of Taar1 influence. Finally, we examine TAAR1-related 3'-5'-cyclic adenosine monophosphate (cAMP) response to MA in B6- and D2-like TAAR1 isoforms.
These studies provide the first evidence that D2 mice possess a Taar1 allele that codes for a non-functional TAAR1, and this allele occurs at high frequency in mice that were bred for higher levels of voluntary MA consumption. The absence of TAAR1 function, as found in D2, Taar1 −/−, and MAHDR mice, increases the risk for MA consumption and decreases the sensitivity to the conditioned aversive and hypothermic effects of MA. These, and published, data suggest that a functional TAAR1 heightens sensitivity to certain aversive and physiological effects of MA that may limit MA use.
MATERIALS AND METHODS
Animals
Methamphetamine drinking selected mouse lines
The MADR mice were selectively bred from an F2 cross of the B6 and D2 inbred strains. Details of the selective breeding procedures and response to selection have been fully described (Shabani et al, 2011; Wheeler et al, 2009). Selective breeding was based on amount of the 40-mg/l MA solution consumed in the drinking procedure described below. Three consecutive pairs of MAHDR and MALDR lines have been created, with comparable outcomes, using these procedures.
Taar1−/− mouse breeding and genotyping
The Taar1 −/− mice were obtained from the U.C. Davis Knockout Mouse Project (KOMP; www.komp.org). Briefly, chimeric mice were created by injecting BALB/c blastocysts with C57BL/6N ES cells in which the entire Taar1 coding region was deleted by homologous recombination using VelociGene’s Null Allele Bac vector. The chimeras were bred with wild-type B6 mice and their offspring genotyped according to the strategy recommended by KOMP using the following primers: ACTCTTCACCAAGAATGTGG (forward); CCAACAGCGCTCAACAGTTC (reverse, wild-type allele); GTCGTCCTAGCTTCCTCACTG (reverse, null allele). Male and female siblings, heterozygous for the targeted locus, were subsequently bred to produce Taar1+/+, +/−, and −/− littermates.
Animal maintenance and housing
Before experiment initiation, mice were group-housed in acrylic plastic shoe-box cages (28 cm × 18 cm × 13 cm; l × w × h), fitted with wire tops. Cages were lined with Bed-O-Cob (The Andersons, Maumee, OH, USA) or ECOFresh bedding (Absorption Corporation, Ferndale, WA). Mice had free access to rodent chow (Purina 5001, 4.5% fat content; Animal Specialties, Hubbard, OR) and water at all times except during testing. Colony room temperature was 20–22 °C, and lights were maintained on a 12 : 12 h light:dark schedule, with lights on at 0600 h. Mice of both sexes were used in all studies. Procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and were approved by the Veterans Affairs Portland Health Care System Institutional Animal Care and Use Committee.
Drugs and Reagents
(+)MA hydrochloride was purchased from Sigma (St Louis, MO, USA) and mixed in tap water for consumption or dissolved in sterile physiological saline (0.9% NaCl; Baxter Healthcare Corporation, Deerfield, IL, USA) for injection. All injections were given intraperitoneally at a volume of 10 ml/kg. N-(3-Ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoromethyl-benzamide (EPPTB) (Liu et al, 2014) was first diluted in DMSO, and subsequently diluted into cAMP assay buffer for a final DMSO concentration of 0.1%.
General Procedures
Taar1 sequencing
Genomic DNA from MALDR and MAHDR mice was extracted from ear or tail tissue using QuickExtract DNA Extraction Solution (Epicentre, Madison, WI). Taar1 DNA was amplified using a Hotstart DNA polymerase kit (Qiagen, Valencia, CA) with sequence specific primers surrounding the SNP-containing region (forward 5′-CACCAACTGGCTCCTTCACT-3′, reverse 5′-CGGTGCTGGTGTGAACTTTA-3′). PCR products were run on a 1.5% agarose gel, and then purified using a QIAquick gel extraction kit (Qiagen, Valencia, CA). Purified DNA was sequenced at the Oregon Health & Science University sequencing core using the forward primer to amplify the Taar1 gene. Sequences of PCR products were aligned and compared with mouse Taar1 sequence (NM_053205.1).
MA two-bottle choice drinking
Procedures were identical to those used during selection of the MADR lines (Shabani et al, 2011; Wheeler et al, 2009). MADR or Taar1 transgenic mice were isolate-housed and habituated for 48 h to drinking from two water-filled drinking tubes. During days 3–10, water was offered vs 20-mg/l MA in water for 4 days, and then 40-mg/l MA in water for 4 days. Mice had 24-h access to water, but only 18-h access to MA. The positions of the water and MA tubes were alternated every 2 days to control for side preferences. Body weight was measured every 2 days and used with volume change to calculate MA consumption in mg/kg. Data from the second and fourth days for each MA concentration (the second day after a change in MA tube position) were averaged to provide a measure of intake.
MA-induced CTA in Taar1 transgenic mice
Identical procedures for MA-induced CTA have been used to test MADR line mice (Shabani et al, 2012b; Wheeler et al, 2009). Briefly, Taar1 +/+, +/−, and −/− mice were isolate-housed and then acclimated to water restriction (2 h of water per day) on days 1–4. On day 5, mice were introduced to the novel taste of a 0.2-M NaCl solution, during a 1-h access period. Conditioning trials then occurred every other day, during which mice were given access for 1 h to the NaCl solution (days 7, 9, 11, 13, and 15), and were then immediately injected with saline or MA (2 mg/kg). This dose of MA was chosen because it induced strong CTA in MALDR mice, but no CTA in MAHDR mice. Water was made available for 30 min, 3 h following injections, to avoid dehydration. On intervening non-conditioning days, water was available for 2 h and no injection was administered.
MA-induced changes in body temperature
MADR or Taar1 transgenic mice were placed in acrylic plastic chambers that isolated the mice from each other and prevented huddling-associated alterations of body temperature (Crabbe et al, 1987, 1989). They were allowed to acclimate for 1 h and then baseline (time 0) rectal temperature was measured (Crabbe et al, 1987). Mice then received an injection of saline or MA (1, 2, 4, 8, or 16 mg/kg for MADR mice; 2 mg/kg for Taar1 mice, based on dose-response results) and were returned to the chambers. Temperatures were recorded at 30, 60, 90, 120, and 180 min after injection. A separate set of MADR mice and Taar1 transgenic mice were identically tested for response to ethanol (with the final reading at 300 min after injection) to examine whether differences in hypothermic response were specific to MA. Doses of ethanol (2 or 4 g/kg) known to induce hypothermia in mice were used (Crabbe et al, 1979).
Cell culture and stable transfection
HEK-293 cells were cultured as we have previously described (Eshleman et al, 1999, 2013). The full-length coding region of the mouse Taar1 (c-terminus GFP tag) cDNA (OriGene, Rockville, MD) was sequenced to verify that it was consistent with the B6 reference. Plasmid DNA was prepared using the Qiagen miniprep kit (Chatwsorth, CA) and Charge Switch Plasmid maxiprep kit (Invitrogen, Grand Island, NY) after transformation of BL-21 competent E. coli cells (Invitrogen). Sequence was verified by EcoR I/Xho I restriction enzyme digestion. The mutation at position 229 in the mouse Taar1 gene (D2-like Taar1) was created using the QuickChange Lightning Kit (Agilent Technologies, Santa Clara, CA) and the B6 sequence. The mutation was verified by sequencing using the VP1.5 primer (5′-GGACTTTCCAAAATGTCG-3′, OriGene). The B6- and D2-like Taar1 expression constructs were transfected into HEK-293 cells using Lipofectamine 2000 (Invitrogen; 10 μg DNA/15 cm plate) according to the manufacturer’s instructions. Stably transfected cells were selected in 600 μg/ml neomycin (G418) and subsequently analyzed for cAMP accumulation in response to MA.
cAMP accumulation assay
Cells expressing the B6- and D2-like constructs were seeded at a density of 2 × 105 cells/well in 48-well tissue culture plates 2 days before an assay, with culture medium containing 10% FCS. One day before the assay, cells were switched to culture medium containing 10% charcoal stripped FCS and incubated overnight. Experiments were completed in assay buffer as previously described (Watts et al, 1998). Seven concentrations of MA (10−8 to 10−4) were added and cells were incubated for 60 min in the presence or absence of 10 μM EPPTB (30 min pre-incubation). cAMP accumulation was measured using a cAMP EIA kit (Cayman Chemical, Ann Arbor MI), according to the manufacturer’s instructions. All experiments were conducted with duplicate determinations.
Confocal microscopy
Cells containing the B6- and D2-like constructs were treated and 0.97 μm intervals were analyzed as we have previously described (Keith et al, 2012).
Immunodetection
Cells were lysed in RIPA lysis buffer containing 1 × protease inhibitor (Roche, San Francisco, CA). Protein concentrations of the samples were determined using a BCA kit (Thermo Scientific, Waltham, MA). Samples were loaded on gels using equal amounts of total protein as we have previously described (Shi and Habecker, 2012). The membrane was incubated at 4 °C overnight with mouse anti-turbo GFP (1 : 2000, OriGene) or β-actin (1 : 2000, Abcam, Cambridge, MA).
Data Analysis
Behavioral and physiological data were analyzed by repeated measures analysis of variance (ANOVA), with selected line, sex, and dose as between-groups factors, and time as a within-subject factor (repeated measure). There were no interactions involving sex and thus, subsequent analyses excluded sex as a factor. Significant two-way interactions were examined using simple main effect analysis, and the Neuman–Keuls test for post hoc mean comparisons was applied, when appropriate. Alpha level was set at 0.05, and statistical analyses were performed using the Statistica 12 software package (StatSoft, Tulsa, OK). The MA dose-response curves for cAMP accumulation were analyzed by ANOVA, with MA dose and receptor type as between-groups factors, followed by Tukey’s test for post hoc mean comparisons. For western blots, immunodetection analysis was carried out using LabWorks software (UVP, Upland, CA). Confocal microscope images were analyzed with LAS AF (Leica Microsystems CMS GmbH, Wetzlar, Germany). Statistical results are presented in the figure legends.
RESULTS
Taar1 Sequence
The D2 strain possesses a non-synonymous allelic variant of the Taar1 gene (Sanger Mouse Genomes Project SNP browser, 2014; Keane et al, 2011; Yalcin et al, 2011), as compared with the reference B6 strain. To date, the SNP (C229A) is reported to be unique to the D2 strain; the reference B6 allele is shared by at least 27 additional strains. The SNP causes a substitution from a proline to a threonine residue at amino-acid position 77 (P77T, Figure 2a), which is situated at the cytoplasmic/luminal interface of the second transmembrane domain. We sequenced the Taar1 gene in the MADR lines and found the D2 allele at a frequency of 1.0 in the 10 MAHDR mice sequenced; every mouse was homozygous for the D2 allele. In the 10 MALDR mice sequenced, both B6 homozygotes and B6/D2 heterozygotes were found (Figure 2b). These data indicate that homozygosity for the D2 allele co-segregates with selection for high MA consumption.
Schematic transmembrane (TM) topology of mouse TAAR1 (adapted from the human TAAR1) and frequency of B6- and D2-like Taar1 alleles in MALDR and MAHDR mice. (a) Amino-acid residues incorporated in the transmembrane domains are shaded in gray. Residues comprising the putative ligand binding vector in locations homologous to human TAAR1 are colored red. N-linked glycosylation at N9, as well as the disulfide bridge linking C95 and C181, are indicated according to the annotation in Uniprot entry Q923Y8_TAAR1_MOUSE. Mouse SNP rs33645709 encodes a non-synonymous proline to threonine mutation at amino-acid position 77 (P77T) in D2, compared with B6 mice. Further details are provided in the text. Figure adapted with permission from Lindemann et al (2005). (b) Frequency of B6 and D2 Taar1 alleles in MALDR and MAHDR mice. Taar1 was sequenced in MALDR and MAHDR mice (n=10/line; replicate 2, selection generation 5). ‘A’ and ‘C’ refer to adenine and cytosine, respectively. MAHDR mice are homozygous for the D2 allele at nucleotide 229. This SNP leads to a threonine at amino-acid position 77. MALDR mice are either homozygous or heterozygous for the B6 allele. B6: C57BL/6J; D2: DBA/2J; MALDR: MA low drinking; MAHDR: MA high drinking.
MA Drinking
Figure 3a shows MA consumption in MADR and Taar1 +/+, +/−, and −/− mice. Shown for comparison is published MA consumption data for the progenitor B6 and D2 strains (Eastwood and Phillips, 2012). Fold changes were calculated to provide an index of magnitude of difference between genotypes. Data were collected in independent experiments and could not be legitimately included in a single statistical analysis. MAHDR mice consumed 9- and 11.9-fold more MA at the 20- and 40-mg/l concentrations (respectively), compared with MALDR mice. D2 mice consumed 3.8- and 6.6-fold more MA than B6 mice at the 20- and 40-mg/l concentrations (Eastwood and Phillips, 2012). Taar1 +/+ and +/− mice consumed only small amounts of MA, and Taar1 −/− mice consumed 3.3- and 6.4-fold more MA at the 20-and 40-mg/l concentrations, compared with Taar1 +/+ mice. The dose consumed by MAHDR, D2, and Taar1 −/− mice was significantly greater when MA was offered as a 40-mg/l concentration.
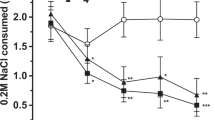
(a) Methamphetamine (MA) consumption differs by Taar1 genotype. DBA/2J (D2) mice consume more MA in mg per kg than C57BL/6J (B6) mice, as previously reported (Eastwood and Phillips, 2012). Data shown here support the greater MA consumption of MAHDR mice, compared with MALDR mice. There was a significant line × concentration interaction (F(1,122)=121.3; p<0.0001); MA intake was higher in MAHDR mice for both MA concentrations, but the difference was greater for 40 mg/l MA. Taar1 −/− mice consumed more MA than Taar1 +/− or +/+ mice. There was a genotype × concentration interaction (F(2,63)=14.4 p<0.001); MA intake differed for both MA concentrations, but the difference was greater for 40 mg/l MA. *p<0.05 for the difference between the lines or genotypes (D2 vs B6, MAHDR vs MALDR, Taar1 −/− vs Taar1 +/+ and Taar1 −/− vs Taar1 +/−) within each concentration. N=62/MADR line (49–54 days old; replicate 3, selection generation 4), and 19–28/transgenic genotype (95–365 days old). B6 and D2 data are shown here with permission (Eastwood and Phillips, 2012). MALDR: MA low drinking; MAHDR: MA high drinking. (b) Sensitivity to MA-induced conditioned taste aversion (CTA) differs by Taar1 genotype. Taar1 −/− mice were insensitive to MA-induced CTA at doses that produce CTA in Taar1 +/+ and +/− mice. There was a significant genotype × treatment × day interaction (F(8,152)=2.7 p<0.01). Subsequent analysis in MA-treated mice identified a significant genotype × day interaction (F(8,80)=4.17 p<0.0005) that was not found in saline-treated mice. Shown are means±SEM. +p<0.05 for the difference between Taar1 −/− vs Taar1 +/+ and Taar1 −/− vs Taar1 +/− on specific day; *p<0.05 for the difference in NaCl consumption on the indicated day, compared with consumption on day 7 before conditioning, within genotype, N=5–8/transgenic genotype for saline, and 7–8/transgenic genotype for MA (109–176 days old).
MA-Induced CTA
MA-induced CTA to the novel NaCl solution was observed in Taar1 +/+ and +/− mice, but not in Taar1 −/− mice. Statistical analyses supported significant reductions in NaCl consumption across conditioning trials, only in the MA-treated Taar1 +/+ and +/− mice (Figure 3b). Saline treatment had no significant effect on NaCl consumption.
Thermal Response to MA
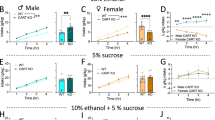
Figure 4a shows the core body temperature response to multiple doses of MA in replicate two MADR mice. MAHDR mice exhibited a hyperthermic response to all doses of MA (1–16 mg/kg), whereas the primary response in MALDR mice was hypothermia. Similar data were generated in replicate three MADR mice (Supplementary Figure 1). Taar1 transgenic mice were subsequently tested with 2 mg/kg MA (Figure 4b), because this dose produced a clear difference in hypothermic response in both replicate sets of MADR mice. Data for MADR mice from Figure 4a are reproduced in Figure 4c to facilitate direct comparison. Taar1 +/+ and +/− mice responded similarly to MALDR mice, showing hypothermia, whereas Taar1 −/− mice did not experience significant hyperthermia or hypothermia. Therefore, the hypothermic response occurred in mice that possess the B6-like Taar1 allele. The difference in sensitivity to the hypothermic effect of MA did not generalize to ethanol, as all genotypes showed hypothermia and there were no genotype-dependent differences (Figures 4d,e).
Methamphetamine (MA)-induced hypothermia differs by Taar1 genotype. (a) MALDR mice exhibited hypothermia in response to 1, 2, and 4 mg/kg MA, whereas MAHDR mice exhibited hyperthermia in response to all MA doses. There was a significant line × time × dose interaction (F(25,800)=3.2; p<0.0001) and subsequent analysis identified a significant line × time interaction at each dose of MA (F’s(5,105–165)=7.5–22.8; all p’s<0.0001). Significant changes in temperature across time were found in both lines of mice at all MA doses, except in MALDR mice at 16 mg/kg MA. Thermal data in saline-treated mice were similar for the MALDR and MAHDR lines, and there were no significant differences in body temperature from baseline, except for a small reduction at 180 min. N=12/MADR line and dose; 62–99 days old; replicate 2, selection generation 5. (b, c) Taar1 +/+, and Taar1 +/− mice exhibit hypothermia in response to 2 mg/kg MA, similar to MALDR mice, whereas the hypothermic response is absent in MAHDR and Taar1 −/− mice. Data for 2 mg/kg MA in MADR mice are shown in (b) to facilitate comparison with Taar1 transgenic mice, and are the same data shown in (a). For the Taar1 transgenic mice (c), there was a significant interaction of genotype × time (F(10,80)=3.3 p<0.005) and significant changes in temperature across time were found for all transgenic genotypes, except Taar1 −/−. N=11–13/transgenic line and dose (92–290 days old). (d, e) Ethanol-induced hypothermia was induced by both 2 and 4 g/kg ethanol in MADR mice and Taar1 transgenic mice and no significant differences were found. (d) For MAHDR and MALDR mice, there was a significant time × dose interaction (F(7,329)=17.1; p<0.0001), with greater hypothermia induced by the higher ethanol dose. N=12–13/MADR line; 86–96 days old; replicate 2, selection generation 5. (e) For Taar1 transgenic mice, there was a significant time × dose interaction (F(7,581)=18.0; p<0.0001), with greater hypothermia induced by the higher ethanol dose. N=12–16/ transgenic line and dose (140–220 days old). Mean comparisons collapsed on genotype identified significant differences in core temperature between the baseline measure and after ethanol treatment. Note: Taar1 mice were in short supply and before testing for ethanol-induced hypothermia, had been included in another study in which MA was given 5 times at a frequency of every 48 h, and then allowed a 2-week rest interval between the studies. Shown are means±SEM. +p<0.05 for the difference between the lines or genotypes; *p<0.05 for the difference between baseline temperature and temperature at a given time point within a given genotype, or in the case of ethanol, for the genotypes collapsed. MADR: MA drinking; MALDR: MA low drinking; MAHDR: MA high drinking.
TAAR1 Function and MA Consuming Mice
To determine differences in function between the B6- and D2-like isoforms of TAAR1, site-directed mutagenesis was used to create the D2 construct found in all MAHDR mice. Both the wild-type and mutant constructs were stably transfected into HEK-293 cells, cells were treated with the TAAR1 agonist, MA, and cAMP accumulation was measured using ELISA. MA elicited a dose-dependent response in cells expressing B6-like TAAR1 (EC50=826 nM), and the effect was blocked by the TAAR1 antagonist, EPPTB (Figure 5). cAMP accumulation was absent following MA treatment of non-transfected cells and of cells expressing D2-like TAAR1, suggesting that the receptor is non-functional. Immunoblot verified that both the functional B6- and the non-functional D2-like receptors were expressed in transfected HEK-293 cells. Confocal microscopic analysis of GFP-tagged constructs corroborated the immunoblot data, and indicated that both forms of TAAR1 are cytosolic, consistent with previous reports (Bunzow et al, 2001; Xie et al, 2007).
The B6-like, but not D2-like, isoform of TAAR1 is activated by MA in Taar1-transfected cells. HEK293 cells were stably transfected with GFP-tagged B6- or D2-like Taar1, and cAMP assays were performed as described in the text. (a) The B6-like isoform of TAAR1 responds to MA stimulation (EC50=826 nM); however, the D2-like isoform does not, suggesting that the receptor is non-functional. Administration of the TAAR1 antagonist EPPTB (EC50~60 μM) produced a right-ward shift in MA-induced cAMP accumulation in the B6-like recombinant TAAR1. Data shown are the average of at least three independent experiments, each conducted with duplicate determinations. Shown are means±SEM. There was a significant dose × receptor type interaction (F(18,56)=23.4; p<0.0001). *p<0.01 for the comparison between the indicated group and untransfected control. +p<0.01 for the comparison between the MA alone and MA+EPPTB C57BL/6J TAAR1 groups. (b) Both the functional B6-like and the non-functional D2-like isoforms of TAAR1 were expressed in transfected cells. Untransfected (Untrans) cells did not express TAAR1. β-Actin was measured as a loading control. (c) Confocal images demonstrating expression of the functional B6-like and non-functional D2-like TAAR1 in cells. B6: C57BL/6J; D2: DBA/2J.
DISCUSSION
In the current experiments, MA drinking, sensitivity to MA-induced CTA, and thermal response to MA corresponded with Taar1 genotype. Homozygous expression of a non-functional isoform of TAAR1 was associated with heightened genetic risk for MA intake. Homo- or heterozygous expression of functional TAAR1 appears to protect against MA consumption, and suggests that the D2 Taar1 polymorphism is not a dominant negative mutation. However, the functional Taar1 allele is dominant in its effect on MA intake. The segregation of the D2-like and B6-like alleles in MAHDR and MALDR mice confirms the direction of allele influence predicted by the QTL on chromosome 10 (Belknap et al, 2013). Overall, these data provide strong support for Taar1 as a candidate gene for regulation of MA consumption. However, the MA consumption phenotypes of the MAHDR and MALDR mice were more extreme than those of the non-selectively bred D2/B6 and transgenic mice, as indicated by the fold-difference data. The influence of other genes is supported by this finding and by the finding that the chromosome 10 QTL accounts for about half, not all, of the genetically determined variance in MA intake.
Lower genetic risk for MA consumption was associated with sensitivity to MA-induced CTA and hypothermia. This outcome was observed in two genetic models. Transgenic mice homozygous or heterozygous for a functional Taar1 allele avoid MA consumption and are sensitive to MA-induced CTA and hypothermia. Similarly, MALDR mice, which are either homozygous or heterozygous for a functional Taar1 allele, avoid MA consumption and are sensitive to aversive effects of MA (Wheeler et al, 2009; Shabani et al, 2011, 2012b) and MA-induced hypothermia. This outcome is clear in two replicate sets of MADR lines, which strongly supports common genetic influence on MA consumption and sensitivity to the aversive and hypothermic effects of MA. Furthermore, combined with data from the Taar1 transgenic mice, these data suggest Taar1 as a candidate gene that influences all three traits. Alternatively, Taar1 may regulate one response that influences the others. For example, Taar1-regulated sensitivity to MA-induced hypothermia may cause a reduction in MA consumption or underlie conditioned aversion. This hypothesis could be tested by preventing the hypothermic effect in MALDR mice while measuring MA intake. MADR mice do not differ in locomotor stimulation to 0.5, 2, or 4 mg/kg MA (Shabani et al, 2011); therefore, genotype-specific differences in hypothermia at these doses of MA are not likely due to differential locomotor activation by MA. However, unpublished data in the MADR lines indicate greater sensitivity of MAHDR mice, like Taar1 −/− mice, to the locomotor stimulant effects of some higher doses of MA. Finally, sensitivity to ethanol-induced hypothermia appears to be regulated by genetic factors distinct from those that influence sensitivity to the thermal effects of MA, as the response to ethanol was similar in both the transgenic mice and MADR mice.
Risk for drug use is affected by the balance of positive and negative experiences with a drug (Cruickshank and Dyer, 2009; Davis and Riley, 2010). Considerable attention has been given to positive rewarding effects associated with MA addiction (Beckmann et al, 2010; Horton et al, 2011; Kamens et al, 2005; Mahler et al, 2013; Meyer et al, 2011; Mizoguchi et al, 2004; Shabani et al, 2011, 2012a; Wheeler et al, 2009), whereas aversive effects that could limit intake have been given less consideration (Harrod et al, 2010; Pringle et al, 2008; Shabani et al, 2011, 2012b; Wheeler et al, 2009). Greater sensitivity to the hyperthermic effects of MA did not correspond with reduced voluntary MA drinking. Instead, heightened sensitivity to MA-induced hypothermia was associated with low MA intake and greater sensitivity to MA-induced aversion. MA is an agonist at TAAR1 (Bunzow et al, 2001; Reese et al, 2014; Wolinsky et al, 2007) and the outcome of hypothermia is in agreement with other reports of TAAR1 agonist-induced hypothermia in rodents (Di Cara et al, 2011; Fantegrossi et al, 2013; Panas et al, 2010; Sabol et al, 2013). Thus, it appears that TAAR1 mediates MA-induced hypothermia and that the immediate hypothermic effect of MA may have a role in curbing MA intake in MALDR, Taar1 +/+ and Taar1 +/− mice. Reduced body temperature alone does not induce CTA in rodents (Misanin et al, 1998). However, hypothermia did prolong the associative period during which aversion could be conditioned (Christianson et al, 2005; Misanin et al, 1998, 2002). Therefore, one possible role of the hypothermic response in MA consumption and MA-induced CTA is that MA-induced hypothermia may increase the association of MA with unpleasant physiological or subjective effects.
MA causes synaptic release of DA and other monoamines, including NE and 5-HT (Fleckenstein et al, 2007; Rothman et al, 2001). MA de-vesicularizes monoamines, which diffuse into the cytoplasm and can then be reverse transported into the synapse (Fleckenstein et al, 2007). Genes encoding the NE transporter (Slc6a2), and the 5-HT transporter (Slc6a4), but not the DA transporter (Slc6a3), are more highly expressed in nucleus accumbens (NAcc) tissue from MAHDR mice than MALDR mice (Wheeler et al, 2009). These genes are not located on mouse chromosome 10, and are therefore not candidates for the QTG in that region (Belknap et al, 2013). However, Taar1 is within the QTL interval, and it modulates monoamine levels by altering transporter function in mice and primates (Miller, 2011, 2012; Revel et al, 2011; Xie and Miller, 2008, 2009). Furthermore, Taar1 −/− mice exhibit lower basal levels and greater amphetamine-induced release of DA in the striatum, compared with +/+ mice (Lindemann et al, 2008; Wolinsky et al, 2007). Similarly, MAHDR mice, which carry the non-functional version of the TAAR1, also exhibit lower resting DA tone in the NAcc and medial prefrontal cortex (mPFC), and higher MA-induced DA release in the mPFC, but not in the NAcc (Lominac et al, 2014). Therefore, DA-related phenotypes may be associated with level of MA intake. On the other hand, differences in 5-HT disposition in MADR and Taar1 transgenic mice do not correspond. Taar1 −/− mice have lower basal levels of 5-HT and greater amphetamine-induced 5-HT release compared with +/+ mice (Wolinsky et al, 2007), whereas the opposite relationship is seen in MADR mice; MAHDR mice have higher basal levels of 5-HT in the NAcc and show reduced sensitivity to MA-induced increases in 5-HT (Lominac et al, 2014). Different brain regions and assay methods could explain discrepancies related to 5-HT. On the other hand, 5-HT may not have a role in genetically-determined differences in MA intake.
Ours is the first report of higher voluntary MA intake in animals with genetic alterations resulting in loss of TAAR1 function. A recent publication involving a pharmacological manipulation of TAAR1 yielded similar results (Jing et al, 2015). The TAAR1 agonist, RO5263397, dose-dependently reduced MA self-administration in rats, just as a functional TAAR1 in our studies was associated with reduced voluntary MA intake. In the study in rats, reinstatement of MA seeking was also attenuated by the TAAR1 agonist, whereas the TAAR1 agonist had no effect on reinstatement of sucrose seeking. MADR mice consume similar amounts of saccharin and quinine, indicating that TAAR1 function in this genetic model does not have a role in the consumption of a natural reward or bitter substance (Shabani et al, 2011; Wheeler et al, 2009). A TAAR1 agonist approach cannot be taken in our mice, because the receptor is non-functional.
The 999-bp mouse Taar1 on chromosome 10 is phylogenetically related to the 1020-bp human TAAR1 on chromosome 6 (Lindemann et al, 2005), and the 332 amino-acid mouse receptor shares 76% homology with the 339 amino-acid human receptor (Borowsky et al, 2001). There are a number of reported synonymous and non-synonymous SNPs in the human TAAR1 (dbSNP NCBI, 2014), but there are no reported polymorphisms that are shared across the mouse and the human. Some of the reported non-synonymous SNPs in the human TAAR1 are located in regions that should alter receptor recognition of ligand or receptor function (Pardo et al, 1992). A non-functional TAAR1 in mice is associated with higher levels of voluntary MA consumption and reduced sensitivity to aversive effects of MA. It is possible that the TAAR1 limits MA consumption in some humans by conferring sensitivity to aversive effects of MA. Therefore, drugs that stimulate a sub-functional TAAR1 may increase sensitivity to aversive effects of MA and be useful for treating MA addiction.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Achat-Mendes C, Lynch LJ, Sullivan KA, Vallender EJ, Miller GM (2012). Augmentation of methamphetamine-induced behaviors in transgenic mice lacking the trace amine-associated receptor 1. Pharmacol Biochem Behav 101: 201–207.
Beckmann JS, Siripurapu KB, Nickell JR, Horton DB, Denehy ED, Vartak A et al (2010). The novel pyrrolidine nor-lobelane analog UKCP-110 [cis-2,5-di-(2-phenethyl)-pyrrolidine hydrochloride] inhibits VMAT2 function, methamphetamine-evoked dopamine release, and methamphetamine self-administration in rats. J Pharmacol Exp Ther 335: 841–851.
Belknap JK, McWeeney S, Reed C, Burkhart-Kasch S, McKinnon CS, Li N et al (2013). Genetic factors involved in risk for methamphetamine intake and sensitization. Mamm Genome 24: 446–458.
Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL et al (2001). Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA 98: 8966–8971.
Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W Jr. et al (1994). Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther 268: 1571–1580.
Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI et al (2001). Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 60: 1181–1188.
Christianson JP, Anderson MJ, Misanin JR, Hinderliter CF (2005). Effect of low body temperature on associative interference in conditioned taste aversion. Percept Mot Skills 100: 913–919.
Crabbe JC, Feller DJ, Dorow JS (1989). Sensitivity and tolerance to ethanol-induced hypothermia in genetically selected mice. J Pharmacol Exp Ther 249: 456–461.
Crabbe JC, Kosobud A, Tam BR, Young ER, Deutsch CM (1987). Genetic selection of mouse lines sensitive (cold) and resistant (hot) to acute ethanol hypothermia. Alcohol Drug Res 7: 163–174.
Crabbe JC, Rigter H, Uijlen J, Strijbos C (1979). Rapid development of tolerance to the hypothermic effect of ethanol in mice. J Pharmacol Exp Ther 208: 128–133.
Cruickshank CC, Dyer KR (2009). A review of the clinical pharmacology of methamphetamine. Addiction 104: 1085–1099.
Davis CM, Riley AL (2010). Conditioned taste aversion learning: implications for animal models of drug abuse. Ann NY Acad Sci 1187: 247–275.
dbSNP NCBI (2014). SNPs in human Taar1 query http://www.ncbi.nlm.nih.gov/snp/.
Di Cara B, Maggio R, Aloisi G, Rivet JM, Lundius EG, Yoshitake T et al (2011). Genetic deletion of trace amine 1 receptors reveals their role in auto-inhibiting the actions of ecstasy (MDMA). J Neurosci 31: 16928–16940.
Eastwood EC, Phillips TJ (2012). Opioid sensitivity in mice selectively bred to consume or not consume methamphetamine. Addict Biol 19: 370–379.
Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A (1999). Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Ther 289: 877–885.
Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A (2013). Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol 85: 1803–1815.
Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC (2013). In vivo effects of abused 'bath salt' constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 38: 563–573.
Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR (2007). New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol 47: 681–698.
Harrod SB, Lacy RT, Ballina LE (2010). Persistent expression of methamphetamine-induced CTA in periadolescent rats. Pharmacol Biochem Behav 96: 515–520.
Horton DB, Siripurapu KB, Norrholm SD, Culver JP, Hojahmat M, Beckmann JS et al (2011). meso-Transdiene analogs inhibit vesicular monoamine transporter-2 function and methamphetamine-evoked dopamine release. J Pharmacol Exp Ther 336: 940–951.
Jing L, Zhang Y, Li JX (2015). Effects of the trace amine-associated receptor 1 agonist RO5263397 on abuse-related behavioral indices of methamphetamine in rats. Int J Neuropsychopharmacol.
Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ (2005). Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav 4: 110–125.
Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B et al (2011). Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477: 289–294.
Keith DJ, Wolfrum K, Eshleman AJ, Janowsky A (2012). Melittin initiates dopamine transporter internalization and recycling in transfected HEK-293 cells. Eur J Pharmacol 690: 13–21.
Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC (2005). Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics 85: 372–385.
Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H et al (2008). Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 324: 948–956.
Liu X, Grandy DK, Janowsky A (2014). Ractopamine, a livestock feed additive, is a full agonist at trace amine-associated receptor 1. J Pharmacol Exp Ther 350: 124–129.
Lominac KD, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Miller BW et al (2014). Mesocorticolimbic monoamine correlates of methamphetamine sensitization and motivation. Front Syst Neurosci 8: 70.
Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M et al (2013). A rodent ‘self-report’ measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behav Brain Res 236: 78–89.
Meyer AC, Horton DB, Neugebauer NM, Wooters TE, Nickell JR, Dwoskin LP et al (2011). Tetrabenazine inhibition of monoamine uptake and methamphetamine behavioral effects: locomotor activity, drug discrimination and self-administration. Neuropharmacology 61: 849–856.
Miller GM (2011). The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem 116: 164–176.
Miller GM (2012). Avenues for the development of therapeutics that target trace amine associated receptor 1 (TAAR1). J Med Chem 55: 1809–1814.
Misanin JR, Anderson MJ, Christianson JP, Collins MM, Goodhart MG, Rushanan SG et al (2002). Low body temperature, time dilation, and long-trace conditioned flavor aversion in rats. Neurobiol Learn Mem 78: 167–177.
Misanin JR, Wilson HA, Schwarz PR, Tuschak JB, Hinderliter CF (1998). Low body temperature affects associative processes in long-trace conditioned flavor aversion. Physiol Behav 65: 581–590.
Mizoguchi H, Yamada K, Mizuno M, Mizuno T, Nitta A, Noda Y et al (2004). Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol Pharmacol 65: 1293–1301.
Panas HN, Lynch LJ, Vallender EJ, Xie Z, Chen GL, Lynn SK et al (2010). Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J Neurosci Res 88: 1962–1969.
Pardo L, Ballesteros JA, Osman R, Weinstein H (1992). On the use of the transmembrane domain of bacteriorhodopsin as a template for modeling the three-dimensional structure of guanine nucleotide-binding regulatory protein-coupled receptors. Proc Natl Acad Sci USA 89: 4009–4012.
Pringle RB, Mouw NJ, Lukkes JL, Forster GL (2008). Amphetamine treatment increases corticotropin-releasing factor receptors in the dorsal raphe nucleus. Neurosci Res 62: 62–65.
Reese EA, Norimatsu Y, Grandy MS, Suchland KL, Bunzow JR, Grandy DK (2014). Exploring the determinants of trace amine-associated receptor 1's functional selectivity for the stereoisomers of amphetamine and methamphetamine. J Med Chem 57: 378–390.
Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R et al (2011). TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci USA 108: 8485–8490.
Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI et al (2001). Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39: 32–41.
Sabol KE, Yancey DM, Speaker HA, Mitchell SL (2013). Methamphetamine and core temperature in the rat: ambient temperature, dose, and the effect of a D2 receptor blocker. Psychopharmacology (Berl) 228: 551–561.
Sanger Mouse Genomes Project SNP browser (2014). Taar1 SNP query. https://www.sanger.ac.uk/resources/mouse/genomes.
Shabani S, Dobbs LK, Ford MM, Mark GP, Finn DA, Phillips TJ (2012a). A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology.
Shabani S, McKinnon CS, Cunningham CL, Phillips TJ (2012b). Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology 62: 1134–1141.
Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ (2011). Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav 10: 625–636.
Shi X, Habecker BA (2012). gp130 cytokines stimulate proteasomal degradation of tyrosine hydroxylase via extracellular signal regulated kinases 1 and 2. J Neurochem 120: 239–247.
Watts VJ, Wiens BL, Cumbay MG, Vu MN, Neve RL, Neve KA (1998). Selective activation of Galphao by D2L dopamine receptors in NS20Y neuroblastoma cells. J Neurosci 18: 8692–8699.
Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A et al (2009). Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav 8: 758–771.
Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P et al (2007). The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav 6: 628–639.
Xie Z, Miller GM (2008). Beta-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: implication for modulatory roles of trace amines in brain. J Pharmacol Exp Ther 325: 617–628.
Xie Z, Miller GM (2009). Trace amine-associated receptor 1 as a monoaminergic modulator in brain. Biochem Pharmacol 78: 1095–1104.
Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ et al (2007). Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharmacol Exp Ther 321: 116–127.
Yalcin B, Wong K, Agam A, Goodson M, Keane TM, Gan X et al (2011). Sequence-based characterization of structural variation in the mouse genome. Nature 477: 326–329.
Acknowledgements
We acknowledge the contribution of Nikki Walter with preparation of genomic data. Additionally, we would like to thank Emily Eastwood, Harue Baba, Jason Erk, Bill Schutzer, and Amy Eshleman for technical support, and David K Grandy for generous provision of Taar1 transgenic breeders and EPPTB. This work was supported by NIH grant T32 DA007262, The Methamphetamine Abuse Research Center (P50 DA018165), the Portland Alcohol Research Center (P60 AA010760), and by a VA Merit Review grant (I01 BX002106) from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, and the VA Research Career Scientist Program. Taar1 transgenic breeders and EPPTB were provided by David K. Grandy, Oregon Health & Science University. The contents of this article may not necessarily represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Harkness, J., Shi, X., Janowsky, A. et al. Trace Amine-Associated Receptor 1 Regulation of Methamphetamine Intake and Related Traits. Neuropsychopharmacol 40, 2175–2184 (2015). https://doi.org/10.1038/npp.2015.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.61
This article is cited by
-
The genetic susceptibility analysis of TAAR1 rs8192620 to methamphetamine and heroin abuse and its role in impulsivity
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Robust aversive effects of trace amine-associated receptor 1 activation in mice
Neuropsychopharmacology (2023)
-
Dopamine dysfunction in stimulant use disorders: mechanistic comparisons and implications for treatment
Molecular Psychiatry (2022)
-
Selective TAAR1 agonists induce conditioned taste aversion
Psychopharmacology (2022)
-
Potential of Ligands for Trace Amine-Associated Receptor 1 (TAAR1) in the Management of Substance Use Disorders
CNS Drugs (2021)