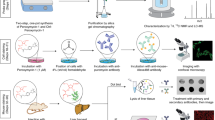
Abstract
During infection, nicotinamide adenine dinucleotide phosphate-oxidase of innate immune cells generates important microbicidal reactive oxygen species (ROS) such as hypochlorous acid (HOCl) to kill the invading pathogens. However, excess amounts of HOCl induce oxidative damage of functional biomolecules such as DNA and proteins, which may cause chronic inflammatory diseases. Herein, we outline protocols for the preparation of a rhodamine-based HOCl probe, as well as applications thereof, with which to detect HOCl in living cells and organisms. The probe (R19S) can be prepared from a commercially available rhodamine, rhodamine 6G, in two steps. When R19S is treated with HOCl, the sulfur atom is replaced by an oxygen atom, resulting in opening of the lactone ring; thus, nonfluorescent R19S is converted to highly fluorescent rhodamine 19 (R19). R19S exhibits high selectivity for HOCl over other ROS and high sensitivity in a weakly acidic environment. In addition, we describe fluorescence imaging assays of HOCl in mouse neutrophils and Drosophila targeted using this probe. The approximate amount of time required to synthesize the probe is 2–3 d, after which it can be used for up to 5 h in the bioimaging of living cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chen, X., Tian, X., Shin, I. & Yoon, J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 40, 4783–4804 (2011).
Nauseef, W.M. Myeloperoxidase in human neutrophil host defense. Cell. Microbiol. 16, 1146–1155 (2014).
Wang, G. & Nauseef, W.M. Salt, chloride, bleach, and innate host defense. J. Leukoc. Biol. 98, 163–172 (2015).
Hampton, M.B., Kettle, A.J. & Winterbourn, C.C. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92, 3007–3017 (1998).
Klebanoff, S.J. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625 (2005).
Clark, R.A., Klebanoff, S.J., Einstein, A.B. & Fefer, A. Peroxidase-H2O2-halide system: cytotoxic effect on mammalian tumor cells. Blood 45, 161–170 (1975).
Jaganjac, M. et al. Granulocytes as effective anticancer agent in experimental solid tumor models. Immunobiology 215, 1015–1020 (2010).
Irani, K. et al. Mitogenic signaling mediated by oxidants in ras-transformed fibroblasts. Science 275, 1649–1652 (1997).
Herdener, M., Heigold, S., Saran, M. & Bauer, G. Target cell-derived superoxide anions cause efficiency and selectivity of intercellular induction of apoptosis. Free Radic. Biol. Med. 29, 1260–1271 (2000).
Prokopowicz, Z.M. et al. Hypochlorous acid: a natural adjuvant that facilitates antigen processing, cross-priming, and the induction of adaptive immunity. J. Immunol. 184, 824–835 (2009).
Andersen, J.K. Oxidative stress in neurodegeneration: cause or consequence? Nat. Med. S18–25 (2004).
Sugiyama, S. et al. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am. J. Pathol. 158, 879–891 (2001).
Hazell, L.J. et al. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Invest. 97, 1535–1544 (1996).
Pattison, D.I. & Davies, M.J. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr. Med. Chem. 13, 3271–3290 (2006).
Winterbourn, C.C. & Kettle, A.J. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free. Radic. Biol. Med. 29, 403–409 (2000).
Yin, J. et al. Preparation of a cyanine-based fluorescent probe for highly selective detection of glutathione and its use in living cells and tissues of mice,. Nat. Protoc. 10, 1742–1754 (2015).
Guo, Z., Kim, G.-H., Yoon, J. & Shin, I. Synthesis of a highly Zn2+-selective cyanine-based probe and its use for tracing endogenous zinc ions in cells and organisms. Nat. Protoc. 9, 1245–1254 (2014).
Xu, Q. et al. Development of imidazoline-2-thiones based two-photon fluorescence probes for imaging hypochlorite generation in a co-culture system. Angew. Chem. Int. Ed. Engl. 54, 4890–4894 (2015).
Zhu, H., Fan, J., Wang, J., Mu, H. & Peng, X. An “Enhanced PET”-based fluorescent probe with ultrasensitivity for imaging basal and elesclomol-induced HClO in cancer cells. J. Am. Chem. Soc. 136, 12820–12823 (2014).
Xu, Q. et al. A highly specific fluorescent probe for hypochlorous acid and its application in imaging microbe-induced HOCl production. J. Am. Chem. Soc. 135, 9944–9949 (2013).
Yuan, L. et al. Development of targetable two-photon fluorescent probes to image hypochlorous acid in mitochondria and lysosome in live cell and inflamed mouse model. J. Am. Chem. Soc. 137, 5930–5938 (2015).
Chen, X. et al. A specific and sensitive method for detection of hypochlorous acid for the imaging of microbe-induced HOCl production. Chem. Commun. 47, 4373–4375 (2011).
Jankowski, A., Scott, C.C. & Grinstein, S. Determinants of the phagosomal pH in neutrophils. J. Biol. Chem. 277, 6059–6066 (2002).
Huynh, K.K. & Grinstein, S. Regulation of vacuolar pH and its modulation by some microbial species. Microbiol. Mol. Biol. Rev. 71, 452–462 (2007).
Lee, K.-A. et al. Bacterial uracil modulates Drosophila DUOX-dependent gut immunity via hedgehog-induced signaling endosomes. Cell Host Microbe 17, 1–14 (2015).
Lee, K.-A. et al. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell 153, 797–811 (2013).
Ha, E.-M. et al. Regulation of DUOX by the Gαq-phospholipase Cβ- Ca2+ pathway in Drosophila gut immunity. Dev. Cell. 16, 386–397 (2009).
Ha, E.-M. et al. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat. Immunol. 10, 949–957 (2009).
Ha, E.-M., Oh, C.-T., Bae, Y.S. & Lee, W.-J. A direct role for dual oxidase in Drosophila gut immunity. Science 310, 847–850 (2005).
Pollock, J.D. et al. Mouse model of X-linked chronic granulomatous disease, and inherited defect in phagocyte superoxide production. Nat. Genet. 9, 202–209 (1995).
Witko-Sarsat, V., Rieu, P., Descamps-latscha, B., Lesavre, P. & Halbwachs-Mecarelli, L. Neutrophils: molecules, functions and pathophusiologica aspects. Lab Invest. 80, 617–653 (2000).
Rosen, H. & Klebanoff, S.J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J. Exp. Med. 149, 27–39 (1979).
Klebanoff, S.J. Myeloperoxidase. Proc. Assoc. Am. Physicians 111, 383–389 (1999).
Wu, S.M. & Pizzo, S.V. α2-Macroglobulin from rheumatid arthritis synovial fluid: functional analysis defines a role for oxidation in inflammation. Arch. Biochem. Biophys. 391, 119–126 (2001).
Hasegawa, T., Malle, E., Farhood, A. & Jaeschke, H. Generation of hypochlorite-modified proteins by neutrophils during ischaemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G760–G767 (2005).
Maruyama, Y., Lindholm, B. & Stenvinkel, P. Inflammation and oxidative stress in ESRD – the role of myeloperoxidase. J. Nephrol. 17, 72–76 (2004).
Bergt, C. et al. Human neutrophils employ the myeloperoxidase/hydrogen peroxide/chloride system to oxidatively damage apolipoprotein A-1. Eur. J. Biochem. 268, 3523–3531 (2001).
Acknowledgements
This work was supported by grants from the National Creative Research Initiative programs of the National Research Foundation of the Ministry of Science, ICT and Future Planning of Korea (2012R1A3A2048814 to J.Y. and 2015R1A3A2033475 to W.-J.L.). K.-A.L. is supported by the Basic Science Research Program (NRF 2013R1A1A2013219), and J.-H.R. is supported by the Korea Mouse Phenotyping Project (2013M3A9D5072550). X.C. acknowledges support from the National Natural Science Foundation of China (21376117) and the Jiangsu Natural Science Funds for Distinguished Young Scholars (BK20140043). We thank E.J. Park (National Cancer Center, Korea) for critical help in MPO mouse work.
Author information
Authors and Affiliations
Contributions
J.Y., W.-J.L. and J.-H.R. designed the experiments; X.C., X.R. and G.K. performed the chemical synthesis and fluorescence assays in vitro; K.-A.L., J.-C.R. and J.-H.R. performed the biological experiments; J.Y., W.-J.L., J.-H.R. and X.C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Note (PDF 91 kb)
Rights and permissions
About this article
Cite this article
Chen, X., Lee, KA., Ren, X. et al. Synthesis of a highly HOCl-selective fluorescent probe and its use for imaging HOCl in cells and organisms. Nat Protoc 11, 1219–1228 (2016). https://doi.org/10.1038/nprot.2016.062
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.062
This article is cited by
-
Design and Synthesis of Novel Fluorescent Probe Based on Cyanobiphenyl and its Application in Detection of Hypochlorite
Journal of Fluorescence (2023)
-
Hypocrates is a genetically encoded fluorescent biosensor for (pseudo)hypohalous acids and their derivatives
Nature Communications (2022)
-
An imine-based colorimetric chemodosimeter for the detection of hypochlorite \((\hbox {ClO}^{-})\) in aqueous media: its application in test strips and real water samples
Journal of Chemical Sciences (2019)
-
Synthesis of an ultrasensitive BODIPY-derived fluorescent probe for detecting HOCl in live cells
Nature Protocols (2018)
-
An NBD–NH2 fluorescent probe for bioimaging: existence of a specific detection of ClO−
Monatshefte für Chemie - Chemical Monthly (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.