Abstract
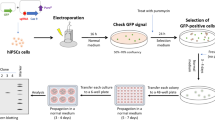
The utility of human induced pluripotent stem cells (iPSCs) is enhanced by an ability to precisely modify a chosen locus with minimal impact on the remaining genome. However, the derivation of gene-edited iPSCs typically involves multiple steps requiring lengthy culture periods and several clonal events. Here, we describe a one-step protocol for reliable generation of clonally derived gene-edited iPSC lines from human fibroblasts in the absence of drug selection or FACS enrichment. Using enhanced episomal-based reprogramming and CRISPR/Cas9 systems, gene-edited and passage-matched unmodified iPSC lines are obtained following a single electroporation of human fibroblasts. To minimize unwanted mutations within the target locus, we use a Cas9 variant that is associated with decreased nonhomologous end-joining (NHEJ) activity. This protocol outlines in detail how this streamlined approach can be used for both monoallelic and biallelic introduction of specific base changes or transgene cassettes in a manner that is efficient, rapid (∼6–8 weeks), and cost-effective.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Chung, C.Y. et al. Identification and rescue of alpha-synuclein toxicity in Parkinson patient-derived neurons. Science 342, 983–987 (2013).
Ryan, S.D. et al. Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell 155, 1351–1364 (2013).
Wang, Y. et al. Genome editing of isogenic human induced pluripotent stem cells recapitulates long QT phenotype for drug testing. J. Am. Coll. Cardiol. 64, 451–459 (2014).
Chen, Y. et al. Engineering human stem cell lines with inducible gene knockout using CRISPR/Cas9. Cell Stem Cell 17, 233–244 (2015).
Gonzalez, F. et al. An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell 15, 215–226 (2014).
Howden, S.E. et al. Simultaneous reprogramming and gene correction of patient fibroblasts. Stem Cell Reports 5, 1109–1118 (2015).
Hou, Z. et al. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 110, 15644–15649 (2013).
Hsu, P.D., Lander, E.S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Ran, F.A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Kwart, D., Paquet, D., Teo, S. & Tessier-Lavigne, M. Precise and efficient scarless genome editing in stem cells using CORRECT. Nat. Protoc. 12, 329–354 (2017).
Hockemeyer, D. & Jaenisch, R. Induced pluripotent stem cells meet genome editing. Cell Stem Cell 18, 573–586 (2016).
Horvath, P. & Barrangou, R. CRISPR/Cas, the immune system of bacteria and archaea. Science 327, 167–170 (2010).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Jiang, F., Zhou, K., Ma, L., Gressel, S. & Doudna, J.A. Structural biology. A Cas9-guide RNA complex preorganized for target DNA recognition. Science 348, 1477–1481 (2015).
Jinek, M. et al. RNA-programmed genome editing in human cells. Elife 2, e00471 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Canver, M.C. et al. Characterization of genomic deletion efficiency mediated by clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 nuclease system in mammalian cells. J. Biol. Chem. 292, 2556 (2017).
Howden, S.E. et al. A Cas9 variant for efficient generation of indel-free knockin or gene-corrected human pluripotent stem cells. Stem Cell Reports 7, 508–517 (2016).
Cho, S.W., Kim, S., Kim, J.M. & Kim, J.S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013).
Merkle, F.T. et al. Efficient CRISPR-Cas9-mediated generation of knockin human pluripotent stem cells lacking undesired mutations at the targeted locus. Cell Rep. 11, 875–883 (2015).
Mao, Z., Bozzella, M., Seluanov, A. & Gorbunova, V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle 7, 2902–2906 (2008).
Saleh-Gohari, N. & Helleday, T. Conservative homologous recombination preferentially repairs DNA double-strand breaks in the S phase of the cell cycle in human cells. Nucleic Acids Res. 32, 3683–3688 (2004).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 (2007).
Forsyth, N.R. et al. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells 8, 16–23 (2006).
Bai, Q. et al. Temporal analysis of genome alterations induced by single-cell passaging in human embryonic stem cells. Stem Cells Dev. 24, 653–662 (2015).
Mahmoudi, S. & Brunet, A. Aging and reprogramming: a two-way street. Curr. Opin. Cell Biol. 24, 744–756 (2012).
Abyzov, A. et al. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature 492, 438–442 (2012).
Gore, A. et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 471, 63–67 (2011).
Howden, S.E. et al. Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc. Natl. Acad. Sci. USA 108, 6537–6542 (2011).
Lo Sardo, V. et al. Influence of donor age on induced pluripotent stem cells. Nat. Biotechnol. 35, 69–74 (2017).
Reinhardt, P. et al. Genetic correction of a LRRK2 mutation in human iPSCs links Parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell 12, 354–367 (2013).
Doench, J.G. et al. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat. Biotechnol. 32, 1262–1267 (2014).
Guschin, D.Y. et al. A rapid and general assay for monitoring endogenous gene modification. Methods Mol. Biol. 649, 247–256 (2010).
Villegas, J. & McPhaul, M. Establishment and culture of human skin fibroblasts. Curr. Protoc. Mol. Biol. Unit 28.23 (2005).
Smithies, O., Gregg, R.G., Boggs, S.S., Koralewski, M.A. & Kucherlapati, R.S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature 317, 230–234 (1985).
Thomas, K.R., Folger, K.R. & Capecchi, M.R. High frequency targeting of genes to specific sites in the mammalian genome. Cell 44, 419–428 (1986).
Chen, F. et al. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat. Methods 8, 753–755 (2011).
Paquet, D. et al. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533, 125–129 (2016).
Kim, J.H. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6, e18556 (2011).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011).
Bae, S., Park, J. & Kim, J.-S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 (2014).
Smith, C. et al. Whole-genome sequencing analysis reveals high specificity of CRISPR/Cas9 and TALEN-based genome editing in human iPSCs. Cell Stem Cell 15, 12–13 (2014).
Crosetto, N. et al. Nucleotide-resolution DNA double-strand breaks mapping by next-generation sequencing. Nat. Methods 10, 361–365 (2013).
Tsai, S.Q. et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 33, 187–197 (2015).
Kim, D. et al. Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat. Methods 12, 237–243 (2015).
Draper, J.S. et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat. Biotechnol. 22, 53–54 (2004).
Cowan, C.A. et al. Derivation of embryonic stem-cell lines from human blastocysts. New Eng. J. Med. 350, 1353–1356 (2004).
Baker, D.E. et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat. Biotechnol. 25, 207–215 (2007).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Park, J., Bae, S. & Kim, J.-S. Cas-Designer: a web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 31, 4014–4016 (2015).
Brinkman, E.K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168 (2014).
Radloff, R., Bauer, W. & Vinograd, J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc. Natl. Acad. Sci. USA 57, 1514–1521 (1967).
Subach, O.M., Cranfill, P.J., Davidson, M.W. & Verkhusha, V.V. An enhanced monomeric blue fluorescent protein with the high chemical stability of the chromophore. PLoS One 6, e28674 (2011).
Takasato, M., Er, P.X., Chiu, H.S. & Little, M.H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 11, 1681–1692 (2016).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Acknowledgements
We acknowledge A. Mallett, Royal Brisbane and Women's Hospital; C. Patel, Genetic Health Queensland; C. Simons and J. Crawford, University of Queensland; B. Bennetts, G. Ho, and K. Holman, Childrens Hospital Westmead; and A. Nandini and team, Pathology Queensland, acting as part of the KidGen Collaborative; for clinical and genetic evaluation, mutation identification, and recruitment of fibroblasts from RG_0120.153 (patient with HNF4A mutation). This work was supported by the National Institutes of Health (DK107344-01) and the National Health and Medical Research Council (NHMRC; GNT1098654 and GNT1100970). M.H.L. is a Senior Principal Research Fellow of the NHMRC (GNT1042093). The Murdoch Children's Research Institute is supported by the Victorian Government's Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Contributions
S.E.H. and J.A.T. conceived and developed the protocol. S.E.H. prepared the manuscript. J.A.T. and M.H.L. provided supervision and assisted in manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
ODNs for sgRNA plasmids used in Anticipated Results section. (PDF 203 kb)
Rights and permissions
About this article
Cite this article
Howden, S., Thomson, J. & Little, M. Simultaneous reprogramming and gene editing of human fibroblasts. Nat Protoc 13, 875–898 (2018). https://doi.org/10.1038/nprot.2018.007
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2018.007
This article is cited by
-
Efficient and safe single-cell cloning of human pluripotent stem cells using the CEPT cocktail
Nature Protocols (2023)
-
Variants in SART3 cause a spliceosomopathy characterised by failure of testis development and neuronal defects
Nature Communications (2023)
-
Large-scale engineering of hiPSC-derived nephron sheets and cryopreservation of their progenitors
Stem Cell Research & Therapy (2022)
-
Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation
Nature Materials (2021)
-
Significant transcriptomic changes are associated with differentiation of bone marrow-derived mesenchymal stem cells into neural progenitor-like cells in the presence of bFGF and EGF
Cell & Bioscience (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.