Abstract
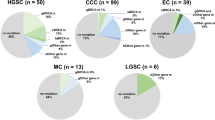
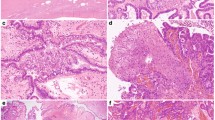
Germline mutation in either BRCA1 or BRCA2 is associated with an increased risk of ovarian cancer, particularly the most common invasive histotype — serous carcinoma. In addition, serous ovarian cancers have an unusually high frequency of other molecular events involving BRCA pathway dysfunction. Recent findings show a high frequency of TP53 mutation, chromosomal instability, distinct molecular subtypes and DNA copy number-driven changes in gene expression. These findings suggest a model in which homologous recombination repair deficiency initiates a cascade of molecular events that sculpt the evolution of high-grade serous ovarian cancer and dictate its response to therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Stewart, B. W. & Kleihues, P. World Cancer Report. (IARC Press, 2003).
Bast, R. C. Jr, Hennessy, B. & Mills, G. B. The biology of ovarian cancer: new opportunities for translation. Nature Rev. Cancer 9, 415–428 (2009).
Jass, J. R. Colorectal cancer: a multipathway disease. Crit. Rev. Oncog. 12, 273–287, (2006).
Silverberg, S. G. Histopathologic grading of ovarian carcinoma: a review and proposal. Int. J. Gynecol. Pathol. 19, 7–15 (2000).
Lee, K. R. & Young, R. H. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am. J. Surg. Pathol. 27, 281–292 (2003).
Zorn, K. K. et al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin. Cancer Res. 11, 6422–6430 (2005).
Schwartz, D. R. et al. Novel candidate targets of β-catenin/T-cell factor signaling identified by gene expression profiling of ovarian endometrioid adenocarcinomas. Cancer Res. 63, 2913–2922 (2003).
Tothill, R. W. et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 14, 5198–5208 (2008).
Kobel, M. et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 5, e232 (2008).
Vang, R., Shih Ie, M. & Kurman, R. J. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 16, 267–282 (2009).
Lee, Y. et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol. 211, 26–35 (2007).
Lee, Y. et al. Advances in the recognition of tubal intraepithelial carcinoma: applications to cancer screening and the pathogenesis of ovarian cancer. Adv. Anat. Pathol. 13, 1–7 (2006).
Folkins, A. K. et al. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol. Oncol. 109, 168–173 (2008).
Crum, C. P. et al. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin. Med. Res. 5, 35–44 (2007).
Tone, A. A. et al. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin. Cancer Res. 14, 4067–4078 (2008).
Marquez, R. T. et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin. Cancer Res. 11, 6116–6126 (2005).
Kindelberger, D. W. et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am. J. Surg. Pathol. 31, 161–169 (2007).
Ahmed, A. A. et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 221, 49–56 (2010).
Risch, H. A. et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J. Natl Cancer Inst. 98, 1694–1706 (2006).
Venkitaraman, A. R. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu. Rev. Pathol. 4, 461–487 (2009).
Geisler, J. P., Hatterman-Zogg, M. A., Rathe, J. A. & Buller, R. E. Frequency of BRCA1 dysfunction in ovarian cancer. J. Natl Cancer Inst. 94, 61–67 (2002).
Hilton, J. L. et al. Inactivation of BRCA1 and BRCA2 in ovarian cancer. J. Natl Cancer Inst. 94, 1396–1406 (2002).
Lim, S. L. et al. Promoter hypermethylation of FANCF and outcome in advanced ovarian cancer. Br. J. Cancer 98, 1452–1456 (2008).
Wang, Z., Li, M., Lu, S., Zhang, Y. & Wang, H. Promoter hypermethylation of FANCF plays an important role in the occurrence of ovarian cancer through disrupting Fanconi anemia-BRCA pathway. Cancer Biol. Ther. 5, 256–260 (2006).
D'Andrea, A. D. The Fanconi Anemia/BRCA signaling pathway: disruption in cisplatin-sensitive ovarian cancers. Cell Cycle 2, 290–292 (2003).
Press, J. Z. et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer 8, 17 (2008).
Jazaeri, A. A. et al. Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers. J. Natl Cancer Inst. 94, 990–1000 (2002).
Patel, K. J. et al. Involvement of BRCA2 in DNA repair. Mol. Cell 1, 347–357 (1998).
Xu, X. et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell 3, 389–395 (1999).
Norquist, B. M. et al. The molecular pathogenesis of hereditary ovarian carcinoma: alterations in the tubal epithelium of women with BRCA1 and BRCA2 mutations. Cancer 27 July 2010 (doi:10.1002/cncr.25439).
Xian, W. et al. The Li-Fraumeni syndrome (LFS): a model for the initiation of p53 signatures in the distal Fallopian tube. J. Pathol. 220, 17–23 (2010).
Levanon, K. et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene 29, 1103–1113 (2010).
Williamson, E. A. et al. BRCA1 and FOXA1 proteins coregulate the expression of the cell cycle-dependent kinase inhibitor p27Kip1. Oncogene 25, 1391–1399 (2006).
Williamson, E. A., Dadmanesh, F. & Koeffler, H. P. BRCA1 transactivates the cyclin-dependent kinase inhibitor p27Kip1. Oncogene 21, 3199–3206 (2002).
Hughes-Davies, L. et al. EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell 115, 523–535 (2003).
Etemadmoghadam, D. et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin. Cancer Res. 15, 1417–1427 (2009).
Gorringe, K. L. et al. Genome-wide survey of copy number associations in ovarian cancer. PLoS ONE (in the press).
Gorringe, K. L. & Campbell, I. G. Large-scale genomic analysis of ovarian carcinomas. Mol. Oncol. 3, 157–164 (2009).
Birrer, M. J. et al. Whole genome oligonucleotide-based array comparative genomic hybridization analysis identified fibroblast growth factor 1 as a prognostic marker for advanced-stage serous ovarian adenocarcinomas. J. Clin. Oncol. 25, 2281–2287 (2007).
Haverty, P. M., Hon, L. S., Kaminker, J. S., Chant, J. & Zhang, Z. High-resolution analysis of copy number alterations and associated expression changes in ovarian tumors. BMC Med. Genomics 2, 21 (2009).
Natrajan, R. et al. Tiling path genomic profiling of grade 3 invasive ductal breast cancers. Clin. Cancer Res. 15, 2711–2722 (2009).
Kobayashi, S. Basal-like subtype of breast cancer: a review of its unique characteristics and their clinical significance. Breast Cancer 15, 153–158 (2008).
Manie, E. et al. High frequency of TP53 mutation in BRCA1 and sporadic basal-like carcinomas but not in BRCA1 luminal breast tumors. Cancer Res. 69, 663–671 (2009).
Holstege, H. et al. High incidence of protein-truncating TP53 mutations in BRCA1-related breast cancer. Cancer Res. 69, 3625–3633 (2009).
Stephens, P. J. et al. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature 462, 1005–1010 (2009).
Pollack, J. R. et al. Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nature Genet. 23, 41–46 (1999).
Lee, H. et al. Mitotic checkpoint inactivation fosters transformation in cells lacking the breast cancer susceptibility gene, BRCA2. Mol. Cell 4, 1–10 (1999).
Merlo, L. M., Pepper, J. W., Reid, B. J. & Maley, C. C. Cancer as an evolutionary and ecological process. Nature Rev. Cancer 6, 924–935 (2006).
van Riggelen, J., Yetil, A. & Felsher, D. W. MYC as a regulator of ribosome biogenesis and protein synthesis. Nature Rev. Cancer 10, 301–309 (2010).
Loeb, K. R. et al. A mouse model for cyclin E-dependent genetic instability and tumorigenesis. Cancer Cell 8, 35–47 (2005).
Spruck, C. H., Won, K. A. & Reed, S. I. Deregulated cyclin E induces chromosome instability. Nature 401, 297–300 (1999).
Flanagan, J. M. et al. DNA methylome of familial breast cancer identifies distinct profiles defined by mutation status. Am. J. Hum. Genet. 86, 420–433 (2010).
Collins, F. S. & Barker, A. D. Mapping the cancer genome. Pinpointing the genes involved in cancer will help chart a new course across the complex landscape of human malignancies. Sci. Am. 296, 50–57 (2007).
The International Cancer Genome Consortium. International network of cancer genome projects. Nature 464, 993–998 (2010).
Mendes-Pereira, A. M. et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol. Med. 1, 315–322 (2009).
Edwards, S. L. et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451, 1111–1115 (2008).
Swisher, E. M. et al. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 68, 2581–2586 (2008).
Sakai, W. et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451, 1116–1120 (2008).
Turner, N., Tutt, A. & Ashworth, A. Hallmarks of 'BRCAness' in sporadic cancers. Nature Rev. Cancer 4, 814–819 (2004).
Fong, P. C. et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 28, 2512–2519 (2010).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Gelmon, K. A. et al. Can we define tumors that will respond to PARP inhibitors? A phase II correlative study of olaparib in advanced serous ovarian cancer and triple-negative breast cancer. J. Clin. Oncol. Abstr. 28, 3002 (2010).
Silver, D. P. et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J. Clin. Oncol. 28, 1145–1153 (2010).
Acknowledgements
Critical feedback during the drafting of this article by the following colleagues was much appreciated: A. Ahmed, K. Alsop, P. Cowin, D. Etemadmoghadam, A. DeFazio, D. Huntsman, J. Sambrook and C. Stewart.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
FURTHER INFORMATION
David D. L. Bowtell's homepage
Rights and permissions
About this article
Cite this article
Bowtell, D. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer 10, 803–808 (2010). https://doi.org/10.1038/nrc2946
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2946
This article is cited by
-
Autophagy modulating therapeutics inhibit ovarian cancer colony generation by polyploid giant cancer cells (PGCCs)
BMC Cancer (2022)
-
6q deletion is frequent but unrelated to patient prognosis in breast cancer
Breast Cancer (2022)
-
Indirect comparison of the diagnostic performance of 18F-FDG PET/CT and MRI in differentiating benign and malignant ovarian or adnexal tumors: a systematic review and meta-analysis
BMC Cancer (2021)
-
CtBP1/2 differentially regulate genomic stability and DNA repair pathway in high-grade serous ovarian cancer cell
Oncogenesis (2021)
-
Lymphovascular space invasion as a prognostic factor of epithelial ovarian cancer: a multicenter study by the FRANCOGYN group
Archives of Gynecology and Obstetrics (2021)