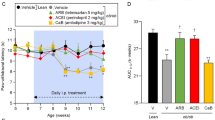
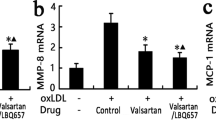
Key Points
-
The approval of LCZ696 for the treatment of heart failure with reduced ejection fraction is the first for chronic neprilysin inhibitor therapy
-
Neprilysin contributes to the formation and degradation of many bioactive peptides and, therefore, chronic neprilysin inhibitor therapy has not only potential benefits but also potential adverse effects
-
By inhibiting the degradation of bradykinin and other inflammatory peptides, neprilysin inhibitor therapy might promote angio-oedema, bronchoconstriction, and inflammation, and might promote cancer by inhibiting the degradation of mitogenic peptides
-
Neprilysin might have a role in metabolizing amyloid-β peptides and by inhibiting amyloid-β degradation, neprilysin inhibition might predispose to diseases related to amyloid-β deposition such as Alzheimer disease, age-related macular degeneration, and cerebral amyloid angiopathy
-
Neprilysin inhibition might also predispose to late-onset sensorimotor axonal polyneuropathy
-
Evidence suggesting potential adverse consequences of chronic neprilysin inhibition come mostly from animal models and whether this evidence applies to humans is unknown; nevertheless, we need to be vigilant in the use of chronic neprilysin inhibitor therapy
Abstract
Neprilysin has a major role in both the generation and degradation of bioactive peptides. LCZ696 (valsartan/sacubitril, Entresto), the first of the new ARNI (dual-acting angiotensin-receptor–neprilysin inhibitor) drug class, contains equimolar amounts of valsartan, an angiotensin-receptor blocker, and sacubitril, a prodrug for the neprilysin inhibitor LBQ657. LCZ696 reduced blood pressure more than valsartan alone in patients with hypertension. In the PARADIGM-HF study, LCZ696 was superior to the angiotensin-converting enzyme inhibitor enalapril for the treatment of heart failure with reduced ejection fraction, and LCZ696 was approved by the FDA for this purpose in 2015. This approval was the first for chronic neprilysin inhibition. The many peptides metabolized by neprilysin suggest many potential consequences of chronic neprilysin inhibitor therapy, both beneficial and adverse. Moreover, LBQ657 might inhibit enzymes other than neprilysin. Chronic neprilysin inhibition might have an effect on angio-oedema, bronchial reactivity, inflammation, and cancer, and might predispose to polyneuropathy. Additionally, inhibition of neprilysin metabolism of amyloid-β peptides might have an effect on Alzheimer disease, age-related macular degeneration, and cerebral amyloid angiopathy. Much of the evidence for possible adverse consequences of chronic neprilysin inhibition comes from studies in animal models, and the relevance of this evidence to humans is unknown. This Review summarizes current knowledge of neprilysin function and possible consequences of chronic neprilysin inhibition that indicate a need for vigilance in the use of neprilysin inhibitor therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
02 February 2017
In the version of this article initially published online, reference 203 was incorrect. The error has been corrected for the HTML, PDF, and print versions of the article.
References
Campbell, D. J. Vasopeptidase inhibition: a double-edged sword? Hypertension 41, 383–389 (2003).
Elsner, D., Müntze, A., Kromer, E. P. & Riegger, G. A. J. Effectiveness of endopeptidase inhibition (candoxatril) in congestive heart failure. Am. J. Cardiol. 70, 494–498 (1992).
Bevan, E. G. et al. Candoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertension. J. Hypertens. 10, 607–613 (1992).
Favrat, B. et al. Neutral endopeptidase versus angiotensin converting enzyme inhibition in essential hypertension. J. Hypertens. 13, 797–804 (1995).
Kentsch, M. et al. Neutral endopeptidase 24.11 inhibition may not exhibit beneficial haemodynamic effects in patients with congestive heart failure. Eur. J. Clin. Pharmacol. 51, 269–272 (1996).
Kostis, J. B. et al. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment versus Enalapril (OCTAVE) trial. Am. J. Hypertens. 17, 103–111 (2004).
Unger, E. F. Approval letter — US Food and Drug Administration. FDA http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/207620Orig1s000ltr.pdf (2015).
McMurray, J. J. et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014).
McMurray, J. J. et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur. J. Heart Fail. 15, 1062–1073 (2013).
Vardeny, O., Miller, R. & Solomon, S. D. Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail. 2, 663–670 (2014).
McKie, P. M. & Burnett, J. C. Jr. Rationale and therapeutic opportunities for natriuretic peptide system augmentation in heart failure. Curr. Heart Fail. Rep. 12, 7–14 (2015).
Minguet, J., Sutton, G., Ferrero, C., Gomez, T. & Bramlage, P. LCZ696: a new paradigm for the treatment of heart failure? Expert Opin. Pharmacother. 16, 435–446 (2015).
Hubers, S. A. & Brown, N. J. Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation 133, 1115–1124 (2016).
Vodovar, N. et al. Neprilysin, cardiovascular, and Alzheimer's diseases: the therapeutic split? Eur. Heart J. 36, 902–905 (2015).
Feldman, A. M., Haller, J. A. & DeKosky, S. T. Valsartan/Sacubitril for heart failure: reconciling disparities between preclinical and clinical investigations. JAMA 315, 25–26 (2016).
Higuchi, Y. et al. Mutations in MME cause an autosomal-recessive Charcot–Marie–Tooth disease type 2. Ann. Neurol. 79, 659–672 (2016).
Auer-Grumbach, M. et al. Rare variants in MME, encoding metalloprotease neprilysin, are linked to late-onset autosomal-dominant axonal polyneuropathies. Am. J. Hum. Genet. 99, 607–623 (2016).
Depondt, C. et al. MME mutation in dominant spinocerebellar ataxia with neuropathy (SCA43). Neurol. Genet. 2, e94 (2016).
Whyteside, A. R. & Turner, A. J. Human neprilysin-2 (NEP2) and NEP display distinct subcellular localisations and substrate preferences. FEBS Lett. 582, 2382–2386 (2008).
Turner, A. J., Brown, C. D., Carson, J. A. & Barnes, K. The neprilysin family in health and disease. Adv. Exp. Med. Biol. 477, 229–240 (2000).
Carpentier, M. et al. Reduced fertility in male mice deficient in the zinc metallopeptidase NL1. Mol. Cell. Biol. 24, 4428–4437 (2004).
Roques, B. P., Noble, F., Daugé, V., Fournié-Zaluski, M.-C. & Beaumont, A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol. Rev. 45, 87–146 (1993).
Shipp, M. A. et al. Common acute lymphoblastic leukemia antigen (CALLA) is active neutral endopeptidase 24.11 (“enkephalinase”): direct evidence by cDNA transfection analysis. Proc. Natl Acad. Sci. USA 86, 297–301 (1989).
Tran-Paterson, R., Boileau, G., Giguere, V. & Letarte, M. Comparative levels of CALLA/neutral endopeptidase on normal granulocytes, leukemic cells, and transfected COS-1 cells. Blood 76, 775–782 (1990).
Kenny, A. J. Endopeptidase-24.11: putative substrates and possible roles. Biochem. Soc. Trans. 21, 663–668 (1993).
Shipp, M. A. et al. CD10/neutral endopeptidase 24.11 hydrolyzes bombesin-like peptides and regulates the growth of small cell carcinomas of the lung. Proc. Natl Acad. Sci. USA 88, 10662–10666 (1991).
Rose, C., Voisin, S., Gros, C., Schwartz, J. C. & Ouimet, T. Cell-specific activity of neprilysin 2 isoforms and enzymic specificity compared with neprilysin. Biochem. J. 363, 697–705 (2002).
Bonvouloir, N., Lemieux, N., Crine, P., Boileau, G. & DesGroseillers, L. Molecular cloning, tissue distribution, and chromosomal localization of MMEL2, a gene coding for a novel human member of the neutral endopeptidase-24.11 family. DNA Cell Biol. 20, 493–498 (2001).
Roques, B. P. et al. The enkephalinase inhibitor thiorphan shows antinociceptive activity in mice. Nature 288, 286–288 (1980).
Turner, A. J. & Murphy, L. J. Molecular pharmacology of endothelin converting enzymes. Biochem. Pharmacol. 51, 91–102 (1996).
Kukkola, P. J. et al. Differential structure–activity relationships of phosphoramidon analogues for inhibition of three metalloproteases: endothelin-converting enzyme, neutral endopeptidase, and angiotensin-converting enzyme. J. Cardiovasc. Pharmacol. 26 (Suppl. 3), S65–S68 (1995).
Loffler, B. M. Endothelin-converting enzyme inhibitors: current status and perspectives. J. Cardiovasc. Pharmacol. 35, S79–S82 (2000).
Northridge, D. B. et al. Effects of UK 69 578: a novel atriopeptidase inhibitor. Lancet 334, 591–593 (1989).
Levin, E. R., Gardner, D. G. & Samson, W. K. Natriuretic peptides. N. Engl. J. Med. 339, 321–328 (1998).
Zois, N. E. et al. Natriuretic peptides in cardiometabolic regulation and disease. Nat. Rev. Cardiol. 11, 403–412 (2014).
Kerkela, R., Ulvila, J. & Magga, J. Natriuretic peptides in the regulation of cardiovascular physiology and metabolic events. J. Am. Heart Assoc. 4, e002423 (2015).
Kuhn, M. Molecular physiology of membrane guanylyl cyclase receptors. Physiol. Rev. 96, 751–804 (2016).
Bhoola, K. D., Figueroa, C. D. & Worthy, K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol. Rev. 44, 1–80 (1992).
Regoli, D., Plante, G. E. & Gobeil, F. Jr. Impact of kinins in the treatment of cardiovascular diseases. Pharmacol. Ther. 135, 94–111 (2012).
Hillmeister, P. et al. Arteriogenesis is modulated by bradykinin receptor signaling. Circ. Res. 109, 524–533 (2011).
Bralet, J. et al. Diuretic and natriuretic responses in rats treated with enkephalinase inhibitors. Eur. J. Pharmacol. 179, 57–64 (1990).
Stasch, J. P., Hirth-Dietrich, C., Ganten, D. & Wegner, M. Renal and antihypertensive effects of neutral endopeptidase inhibition in transgenic rats with an extra renin gene. Am. J. Hypertens. 9, 795–802 (1996).
Campbell, D. J. et al. Effects of neutral endopeptidase inhibition and combined angiotensin converting enzyme and neutral endopeptidase inhibition on angiotensin and bradykinin peptides in rats. J. Pharmacol. Exp. Ther. 287, 567–577 (1998).
Abassi, Z. A., Tate, J. E., Golomb, E. & Keiser, H. R. Role of neutral endopeptidase in the metabolism of endothelin. Hypertension 20, 89–95 (1992).
Smits, G. J., McGraw, D. E. & Trapani, A. J. Interaction of ANP and bradykinin during endopeptidase 24.11 inhibition: renal effects. Am. J. Physiol. Renal Physiol. 258, F1417–F1424 (1990).
Ura, N. et al. The role of kinins and atrial natriuretic peptide on the renal effects of neutral endopeptidase inhibitor in rats. Clin. Exp. Hypertens. 16, 799–808 (1994).
Helin, K., Tikkanen, I., Hohenthal, U. & Fyhrquist, F. Inhibition of either angiotensin-converting enzyme or neutral endopeptidase induces both enzymes. Eur. J. Pharmacol. 264, 135–141 (1994).
Jardine, A. G. et al. The atriopeptidase inhibitor UK 69,578 increases atrial natriuretic factor and causes a natriuresis in normal humans. Am. J. Hypertens. 3, 661–667 (1990).
Ferro, C. J., Spratt, J. C., Haynes, W. G. & Webb, D. J. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation 97, 2323–2330 (1998).
Gros, C. et al. Protection of atrial natriuretic factor against degradation: diuretic and natriuretic responses after in vivo inhibition of enkephalinase (EC 3.4.24.11) by acetorphan. Proc. Natl Acad. Sci. USA 86, 7580–7584 (1989).
Schmitt, F. et al. Acute renal effects of neutral endopeptidase inhibition in humans. Am. J. Physiol. Renal Physiol. 267, F20–F27 (1994).
Richards, A. M. et al. Prolonged inhibition of endopeptidase 24.11 in normal man: renal, endocrine and haemodynamic effects. J. Hypertens. 9, 955–962 (1991).
O'Connell, J. E., Jardine, A. G., Davies, D. L., McQueen, J. & Connell, J. M. Renal and hormonal effects of chronic inhibition of neutral endopeptidase (EC 3.4.24.11) in normal man. Clin. Sci. (Lond.) 85, 19–26 (1993).
Ando, S., Rahman, M. A., Butler, G. C., Senn, B. L. & Floras, J. S. Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension 26, 1160–1166 (1995).
Seymour, A. A. et al. Possible regulation of atrial natriuretic factor by neutral endopeptidase 24.11 and clearance receptors. J. Pharmacol. Exp. Ther. 256, 1002–1009 (1991).
Monopoli, A., Ongini, E., Cigola, E. & Olivetti, G. The neutral endopeptidase inhibitor, SCH 34826, reduces left ventricular hypertrophy in spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 20, 496–504 (1992).
Roksnoer, L. C. et al. Optimum AT1 receptor-neprilysin inhibition has superior cardioprotective effects compared with AT1 receptor blockade alone in hypertensive rats. Kidney Int. 88, 109–120 (2015).
Takeda, Y. et al. Effects of chronic neutral endopeptidase inhibition in rats with cyclosporine-induced hypertension. J. Hypertens. 18, 927–933 (2000).
Richards, A. M. et al. Acute inhibition of endopeptidase 24.11 in essential hypertension: SCH 34826 enhances atrial natriuretic peptide and natriuresis without lowering blood pressure. J. Cardiovasc. Pharmacol. 20, 735–741 (1992).
Richards, A. M. et al. Endopeptidase 24.11 inhibition by SCH 42495 in essential hypertension. Hypertension 22, 119–126 (1993).
Richards, A. M., Crozier, I. G., Espiner, E. A., Yandle, T. G. & Nicholls, M. G. Plasma brain natriuretic peptide and endopeptidase 24.11 inhibition in hypertension. Hypertension 22, 231–236 (1993).
Richards, A. M. et al. Chronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin II. J. Hypertens. 11, 407–416 (1993).
Barber, M. N., Kanagasundaram, M., Anderson, C. R., Burrell, L. M. & Woods, R. L. Vascular neutral endopeptidase inhibition improves endothelial function and reduces intimal hyperplasia. Cardiovasc. Res. 71, 179–188 (2006).
Kugiyama, K. et al. Suppression of atherosclerotic changes in cholesterol-fed rabbits treated with an oral inhibitor of neutral endopeptidase 24.11 (EC 3.4.24.11). Arterioscler. Thromb. Vasc. Biol. 16, 1080–1087 (1996).
Grantham, J. A. et al. Modulation of functionally active endothelin-converting enzyme by chronic neutral endopeptidase inhibition in experimental atherosclerosis. Circulation 101, 1976–1981 (2000).
Goodman, O. B. Jr et al. Neprilysin inhibits angiogenesis via proteolysis of fibroblast growth factor-2. J. Biol. Chem. 281, 33597–33605 (2006).
Winter, R. J., Zhao, L., Krausz, T. & Hughes, J. M. Neutral endopeptidase 24.11 inhibition reduces pulmonary vascular remodeling in rats exposed to chronic hypoxia. Am. Rev. Respir. Dis. 144, 1342–1346 (1991).
Stewart, A. G., Sheedy, W., Thompson, J. S. & Morice, A. H. Effects of SCH 34826, a neutral endopeptidase inhibitor, on hypoxic pulmonary vascular remodelling. Pulm. Pharmacol. 5, 111–114 (1992).
Klinger, J. R. et al. Neutral endopeptidase inhibition attenuates development of hypoxic pulmonary hypertension in rats. J. Appl. Physiol. 75, 1615–1623 (1993).
Thompson, J. S., Sheedy, W. & Morice, A. H. Effects of the neutral endopeptidase inhibitor, SCH 42495, on the cardiovascular remodelling secondary to chronic hypoxia in rats. Clin. Sci. (Lond.) 87, 109–114 (1994).
Schriefer, J. A., Broudy, E. P. & Hassen, A. H. Endopeptidase inhibitors decrease myocardial ischemia/reperfusion injury in an in vivo rabbit model. J. Pharmacol. Exp. Ther. 278, 1034–1039 (1996).
Yang, X. P., Liu, Y. H., Peterson, E. & Carretero, O. A. Effect of neutral endopeptidase 24.11 inhibition on myocardial ischemia/reperfusion injury: the role of kinins. J. Cardiovasc. Pharmacol. 29, 250–256 (1997).
Piedimonte, G., Nadel, J. A., Long, C. S. & Hoffman, J. I. E. Neutral endopeptidase in the heart: neutral endopeptidase inhibition prevents isoproterenol-induced myocardial hypoperfusion in rats by reducing bradykinin degradation. Circ. Res. 75, 770–779 (1994).
Yoshida, K., Yasujima, M., Casley, D. J. & Johnston, C. I. Effect of chronic neutral endopeptidase inhibition on cardiac hypertrophy after experimental myocardial infarction. Jpn Circ. J. 62, 680–686 (1998).
Duncan, A. M. et al. Interaction between neutral endopeptidase and angiotensin converting enzyme inhibition in rats with myocardial infarction: effects on cardiac hypertrophy and angiotensin and bradykinin peptide levels. J. Pharmacol. Exp. Ther. 289, 295–303 (1999).
Cavero, P. G. et al. Cardiorenal actions of neutral endopeptidase inhibition in experimental congestive heart failure. Circulation 82, 196–201 (1990).
Seymour, A. A. et al. Systemic hemodynamics, renal function and hormonal levels during inhibition of neutral endopeptidase 3.4.24.11 and angiotensin-converting enzyme in conscious dogs with pacing-induced heart failure. J. Pharmacol. Exp. Ther. 266, 872–883 (1993).
Rademaker, M. T. et al. Neutral endopeptidase inhibition: augmented atrial and brain natriuretic peptide, haemodynamic and natriuretic responses in ovine heart failure. Clin. Sci. (Lond.) 91, 283–291 (1996).
Margulies, K. B., Barclay, P. L. & Burnett, J. C. Jr. The role of neutral endopeptidase in dogs with evolving congestive heart failure. Circulation 91, 2036–2042 (1995).
Martin, F. L. et al. Natriuretic and antialdosterone actions of chronic oral NEP inhibition during progressive congestive heart failure. Kidney Int. 67, 1723–1730 (2005).
Mishima, T. et al. Effects of chronic neutral endopeptidase inhibition on the progression of left ventricular dysfunction and remodeling in dogs with moderate heart failure. Cardiovasc. Drugs Ther. 16, 209–214 (2002).
Willenbrock, R., Scheuermann, M., Höhnel, K., Luft, F. C. & Dietz, R. Acute and chronic neutral endopeptidase inhibition in rats with aortocaval shunt. Hypertension 27, 1259–1266 (1996).
Munzel, T. et al. Neurohormonal inhibition and hemodynamic unloading during prolonged inhibition of ANF degradation in patients with severe chronic heart failure. Circulation 86, 1089–1098 (1992).
McDowell, G. et al. The effect of the neutral endopeptidase inhibitor drug, candoxatril, on circulating levels of two of the most potent vasoactive peptides. Br. J. Clin. Pharmacol. 43, 329–332 (1997).
Huttenrauch, M. et al. Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer's disease. J. Alzheimers Dis. 44, 1291–1302 (2015).
Walther, T. et al. Improved learning and memory in aged mice deficient in amyloid ß-degrading neutral endopeptidase. PLoS ONE 4, e4590 (2009).
Salles, G., Rodewald, H. R., Chin, B. S., Reinherz, E. L. & Shipp, M. A. Inhibition of CD10/neutral endopeptidase 24.11 promotes B-cell reconstitution and maturation in vivo. Proc. Natl Acad. Sci. USA 90, 7618–7622 (1993).
Wayman, C. P., Baxter, D., Turner, L., Van Der Graaf, P. H. & Naylor, A. M. UK-414,495, a selective inhibitor of neutral endopeptidase, potentiates pelvic nerve-stimulated increases in female genital blood flow in the anaesthetized rabbit. Br. J. Pharmacol. 160, 51–59 (2010).
Dussaule, J. C. et al. Inhibition of neutral endopeptidase stimulates renal sodium excretion in patients with chronic renal failure. Clin. Sci. (Lond.) 84, 31–39 (1993).
Lipkin, G. W., Thuraisingham, R., Dawnay, A. B. S., Harwood, S. M. & Raine, A. E. G. Acute reversal of cyclosporine nephrotoxicity by neutral endopeptidase inhibition in stable renal transplant recipients. Transplantation 64, 1007–1017 (1997).
Wolfensberger, T. J. et al. Evidence for a new role of natriuretic peptides: control of intraocular pressure. Br. J. Ophthalmol. 78, 446–448 (1994).
Hamza, H., Ben Khalifa, H., Baumer, P., Berard, H. & Lecomte, J. M. Racecadotril versus placebo in the treatment of acute diarrhoea in adults. Aliment. Pharmacol. Ther. 13 (Suppl. 6), 15–19 (1999).
Packer, M. et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 106, 920–926 (2002).
Armstrong, P. W., Lorell, B. H., Nissen, S. & Borer, J. Omapatrilat. Circulation 106, e9011–e9012 (2002).
Benz, J. R. et al. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind, placebo controlled trial comparing combination therapy with monotherapy. J. Hum. Hypertens. 12, 861–866 (1998).
Hunyady, L. & Catt, K. J. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 20, 953–970 (2006).
Wadei, H. M. & Textor, S. C. The role of the kidney in regulating arterial blood pressure. Nat. Rev. Nephrol. 8, 602–609 (2012).
Karnik, S. S. et al. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli. Pharmacol. Rev. 67, 754–819 (2015).
Good, J. M. et al. Elevated plasma endothelin concentrations in heart failure; an effect of angiotensin II? Eur. Heart J. 15, 1634–1640 (1994).
Moreau, P. et al. Angiotensin II increases tissue endothelin and induces vascular hypertrophy — reversal by ETA-receptor antagonist. Circulation 96, 1593–1597 (1997).
Richards, A. M. et al. Effect of inhibition of endopeptidase 24.11 on responses to angiotensin II in human volunteers. Circ. Res. 71, 1501–1507 (1992).
Jones, E. S., Vinh, A., McCarthy, C. A., Gaspari, T. A. & Widdop, R. E. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol. Ther. 120, 292–316 (2008).
Peluso, A. A., Santos, R. A., Unger, T. & Steckelings, U. M. The angiotensin type 2 receptor and the kidney. Curr. Opin. Nephrol. Hypertens. http://dx.doi.org/10.1097/MNH.0000000000000289 (2016).
Padia, S. H., Howell, N. L., Siragy, H. M. & Carey, R. M. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension 47, 537–544 (2006).
Koid, S. S., Ziogas, J. & Campbell, D. J. Aliskiren reduces myocardial ischemia-reperfusion injury by a bradykinin B2 receptor- and angiotensin AT2 receptor-mediated mechanism. Hypertension 63, 768–773 (2014).
Abassi, Z. A., Kelly, G., Golomb, E., Klein, H. & Keiser, H. R. Losartan improves the natriuretic response to ANF in rats with high-output heart failure. J. Pharmacol. Exp. Ther. 268, 224–230 (1994).
Campbell, D. J., Krum, H. & Esler, M. D. Losartan increases bradykinin levels in hypertensive humans. Circulation 111, 315–320 (2005).
Jalowy, A., Schulz, R., Dorge, H., Behrends, M. & Heusch, G. Infarct size reduction by AT1-receptor blockade through a signal cascade of AT2-receptor activation, bradykinin and prostaglandins in pigs. J. Am. Coll. Cardiol. 32, 1787–1796 (1998).
Sato, M. et al. Myocardial protection by preconditioning of heart with losartan, an angiotensin II type 1-receptor blocker: Implication of bradykinin-dependent and bradykinin-independent mechanisms. Circulation 102 (Suppl. 3), III-346–III-351 (2000).
Messadi-Laribi, E. et al. Tissue kallikrein is involved in the cardioprotective effect of AT1-receptor blockade in acute myocardial ischemia. J. Pharmacol. Exp. Ther. 323, 210–216 (2007).
Cruden, N. L., Witherow, F. N., Webb, D. J., Fox, K. A. & Newby, D. E. Bradykinin contributes to the systemic hemodynamic effects of chronic angiotensin-converting enzyme inhibition in patients with heart failure. Arterioscler. Thromb. Vasc. Biol. 24, 1043–1048 (2004).
Ménard, J., Campbell, D. J., Azizi, M. & Gonzales, M.-F. Synergistic effects of ACE inhibition and Ang II antagonism on blood pressure, cardiac weight, and renin in spontaneously hypertensive rats. Circulation 96, 3072–3078 (1997).
Ferrario, C. M., Chappell, M. C., Dean, R. H. & Iyer, S. N. Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. J. Am. Soc. Nephrol. 9, 1716–1722 (1998).
Trask, A. J. & Ferrario, C. M. Angiotensin-(1–7): pharmacology and new perspectives in cardiovascular treatments. Cardiovasc. Drug Rev. 25, 162–174 (2007).
Ferrario, C. M., Chappell, M. C., Tallant, E. A., Brosnihan, K. B. & Diz, D. I. Counterregulatory actions of angiotensin-(1–7). Hypertension 30, 535–541 (1997).
Campbell, D. J. in Angiotensin II Receptor Antagonists (eds Epstein, M. & Brunner, H. R.) 9–27 (Hanley and Belfus, 2001).
Loot, A. E. et al. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation 105, 1548–1550 (2002).
Kedzierski, R. M. & Yanagisawa, M. Endothelin system: the double-edged sword in health and disease. Annu. Rev. Pharmacol. Toxicol. 41, 851–876 (2001).
Ksander, G. M. et al. Dicarboxylic acid dipeptide neutral endopeptidase inhibitors. J. Med. Chem. 38, 1689–1700 (1995).
Gu, J. et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J. Clin. Pharmacol. 50, 401–414 (2010).
Criscione, L. et al. Pharmacological profile of valsartan: a potent, orally active, nonpeptide antagonist of the angiotensin II AT1-receptor subtype. Br. J. Pharmacol. 110, 761–771 (1993).
Brown, P. C. Center for Drug Evaluation and Research. Application Number: 207620Orig1s000. Pharmacology Review. FDA http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207620Orig1s000PharmR.pdf (2015).
US Food and Drug Administration. Entresto prescribing information. FDA http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207620Orig1s000lbl.pdf (2015).
Müller, P. et al. Angiotensin II receptor blockade with single doses of valsartan in healthy, normotensive subjects. Eur. J. Clin. Pharmacol. 47, 231–245 (1994).
Langenickel, T. H. et al. The effect of LCZ696 (sacubitril/valsartan) on amyloid-beta concentrations in cerebrospinal fluid in healthy subjects. Br. J. Clin. Pharmacol. 81, 878–890 (2016).
Kobalava, Z. et al. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ696) in patients with heart failure and reduced ejection fraction. Cardiovasc. Ther. 34, 191–198 (2016).
Ruilope, L. M. et al. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 375, 1255–1266 (2010).
Roksnoer, L. C. et al. Blood pressure-independent renoprotection in diabetic rats treated with AT1 receptor-neprilysin inhibition compared with AT1 receptor blockade alone. Clin. Sci. (Lond.) 130, 1209–1220 (2016).
Hegde, L. G. et al. Concomitant angiotensin AT1 receptor antagonism and neprilysin inhibition produces omapatrilat-like antihypertensive effects without promoting tracheal plasma extravasation in the rat. J. Cardiovasc. Pharmacol. 57, 495–504 (2011).
Kusaka, H. et al. LCZ696, angiotensin II receptor-neprilysin inhibitor, ameliorates high-salt-induced hypertension and cardiovascular injury more than valsartan alone. Am. J. Hypertens. 28, 1409–1417 (2015).
Kario, K. et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension 63, 698–705 (2014).
Solomon, S. D. et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 380, 1387–1395 (2012).
von Lueder, T. G. et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ. Heart Fail. 8, 71–78 (2015).
Suematsu, Y. et al. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur. J. Heart Fail. 18, 386–393 (2016).
Bai, H. Y. et al. Pre-treatment with LCZ696, an orally active angiotensin receptor neprilysin inhibitor, prevents ischemic brain damage. Eur. J. Pharmacol. 762, 293–298 (2015).
Lu, B. et al. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat. Med. 3, 904–907 (1997).
Nussberger, J. et al. Plasma bradykinin in angio-oedema. Lancet 351, 1693–1697 (1998).
Nussberger, J., Cugno, M. & Cicardi, M. Bradykinin-mediated angioedema. N. Engl. J. Med. 347, 621–622 (2002).
Sulpizio, A. C. et al. Mechanism of vasopeptidase inhibitor-induced plasma extravasation: comparison of omapatrilat and the novel neutral endopeptidase 24.11/angiotensin-converting enzyme inhibitor GW796406. J. Pharmacol. Exp. Ther. 315, 1306–1313 (2005).
Fryer, R. M. et al. Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br. J. Pharmacol. 153, 947–955 (2008).
Dahlof, B. et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359, 995–1003 (2002).
Dickstein, K. & Kjekshus, J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Lancet 360, 752–760 (2002).
The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 342, 145–153 (2000).
The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker versus diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 288, 2981–2997 (2002).
Pfeffer, M. A. et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N. Engl. J. Med. 349, 1893–1906 (2003).
Duncan, A.-M. et al. Kinins in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R897–R904 (2000).
Campbell, D. J. The kallikrein–kinin system in humans. Clin. Exp. Pharmacol. Physiol. 28, 1060–1065 (2001).
Rubinstein, I., Muns, G. & Zucker, I. H. Plasma exudation in conscious dogs with experimental heart failure. Basic Res. Cardiol. 89, 487–498 (1994).
Koehne, P., Schaper, C., Graf, K. & Kunkel, G. Neutral endopeptidase 24.11: its physiologic and possibly pathophysiologic role in inflammation with special effect on respiratory inflammation. Allergy 53, 1023–1042 (1998).
Stimler-Gerard, N. P. Neutral endopeptidase-like enzyme controls the contractile activity of substance P in guinea pig lung. J. Clin. Invest. 79, 1819–1825 (1987).
Dusser, D. J., Nadel, J. A., Sekizawa, K., Graf, P. D. & Borson, D. B. Neutral endopeptidase and angiotensin converting enzyme inhibitors potentiate kinin-induced contraction of ferret trachea. J. Pharmacol. Exp. Ther. 244, 531–536 (1988).
Dusser, D. J., Djokic, T. D., Borson, D. B. & Nadel, J. A. Cigarette smoke induces bronchoconstrictor hyperresponsiveness to substance P and inactivates airway neutral endopeptidase in the guinea pig. Possible role of free radicals. J. Clin. Invest. 84, 900–906 (1989).
Cheung, D. et al. Neutral endopeptidase activity and airway hyperresponsiveness to neurokinin A in asthmatic subjects in vivo. Am. Rev. Respir. Dis. 148, 1467–1473 (1993).
Crimi, N. et al. Inhibition of neutral endopeptidase potentiates bronchoconstriction induced by neurokinin A in asthmatic patients. Clin. Exp. Allergy 24, 115–120 (1994).
Crimi, N. et al. Effect of an inhaled neutral endopeptidase inhibitor, phosphoramidon, on baseline airway calibre and bronchial responsiveness to bradykinin in asthma. Thorax 50, 505–510 (1995).
Diamant, Z., Van der Veen, H., Kuijpers, E. A. P., Bakker, P. F. & Sterk, P. J. The effect of inhaled thiorphan on allergen-induced airway responses in asthmatic subjects. Clin. Exp. Allergy 26, 525–532 (1996).
de Gouw, H. W., Diamant, Z., Kuijpers, E. A., Sont, J. K. & Sterk, P. J. Role of neutral endopeptidase in exercise-induced bronchoconstriction in asthmatic subjects. J. Appl. Physiol. 81, 673–678 (1996).
Angus, R. M., McCallum, M. J., Nally, J. E. & Thomson, N. C. No effect of the oral neutral endopeptidase inhibitor candoxatril, on bronchomotor tone and histamine reactivity in asthma. Eur. Respir. J. 7, 1084–1089 (1994).
Fischer, H. S. et al. Neutral endopeptidase knockout induces hyperalgesia in a model of visceral pain, an effect related to bradykinin and nitric oxide. J. Mol. Neurosci. 18, 129–134 (2002).
Kramer, H. H., He, L., Lu, B., Birklein, F. & Sommer, C. Increased pain and neurogenic inflammation in mice deficient of neutral endopeptidase. Neurobiol. Dis. 35, 177–183 (2009).
Lu, B. et al. Neutral endopeptidase modulation of septic shock. J. Exp. Med. 181, 2271–2275 (1995).
Connelly, J. C., Skidgel, R. A., Schulz, W. W., Johnson, A. R. & Erdos, E. G. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc. Natl Acad. Sci. USA 82, 8737–8741 (1985).
Shipp, M. A. et al. Downregulation of enkephalin-mediated inflammatory responses by CD10/neutral endopeptidase 24.11. Nature 347, 394–396 (1990).
Sturiale, S. et al. Neutral endopeptidase (EC 3.4.24.11) terminates colitis by degrading substance P. Proc. Natl Acad. Sci. USA 96, 11653–11658 (1999).
Kirkwood, K. S. et al. Deletion of neutral endopeptidase exacerbates intestinal inflammation induced by Clostridium difficile toxin A. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G544–G551 (2001).
Barbara, G. et al. Neutral endopeptidase (EC 3.4.24.11) downregulates the onset of intestinal inflammation in the nematode infected mouse. Gut 52, 1457–1464 (2003).
Koh, Y. H., Moochhala, S. & Bhatia, M. The role of neutral endopeptidase in caerulein-induced acute pancreatitis. J. Immunol. 187, 5429–5439 (2011).
Day, A. L. et al. Neutral endopeptidase determines the severity of pancreatitis-associated lung injury. J. Surg. Res. 128, 21–27 (2005).
Scholzen, T. E. et al. Neutral endopeptidase terminates substance P-induced inflammation in allergic contact dermatitis. J. Immunol. 166, 1285–1291 (2001).
Kramer, H. H. et al. Inhibition of neutral endopeptidase (NEP) facilitates neurogenic inflammation. Exp. Neurol. 195, 179–184 (2005).
Kiemer, A. K. & Vollmar, A. M. The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages. Ann. Rheum. Dis. 60 (Suppl. 3), iii68–iii70 (2001).
Cattaruzza, F., Poole, D. P. & Bunnett, N. W. Arresting inflammation: contributions of plasma membrane and endosomal signalling to neuropeptide-driven inflammatory disease. Biochem. Soc. Trans. 41, 137–143 (2013).
Sumitomo, M., Shen, R. & Nanus, D. M. Involvement of neutral endopeptidase in neoplastic progression. Biochim. Biophys. Acta 1751, 52–59 (2005).
Papandreou, C. N. et al. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat. Med. 4, 50–57 (1998).
Smollich, M. et al. On the role of endothelin-converting enzyme-1 (ECE-1) and neprilysin in human breast cancer. Breast Cancer Res. Treat. 106, 361–369 (2007).
Shen, R. et al. Androgen-induced growth inhibition of androgen receptor expressing androgen-independent prostate cancer cells is mediated by increased levels of neutral endopeptidase. Endocrinology 141, 1699–1704 (2000).
Dai, J. et al. Tumor-suppressive effects of neutral endopeptidase in androgen-independent prostate cancer cells. Clin. Cancer Res. 7, 1370–1377 (2001).
Horiguchi, A. et al. Lentiviral vector neutral endopeptidase gene transfer suppresses prostate cancer tumor growth. Cancer Gene Ther. 14, 583–589 (2007).
Horiguchi, A. et al. Neutral endopeptidase inhibits prostate cancer tumorigenesis by reducing FGF-2-mediated angiogenesis. Prostate Cancer Prostatic Dis. 11, 79–87 (2008).
Iida, K., Zheng, R., Shen, R. & Nanus, D. M. Adenoviral neutral endopeptidase gene delivery in combination with paclitaxel for the treatment of prostate cancer. Int. J. Oncol. 41, 1192–1198 (2012).
Osman, I. et al. Loss of neutral endopeptidase and activation of protein kinase B (Akt) is associated with prostate cancer progression. Cancer 107, 2628–2636 (2006).
Terauchi, M. et al. Anti-progressive effect of neutral endopeptidase 24.11 (NEP/CD10) on cervical carcinoma in vitro and in vivo. Oncology 69, 52–62 (2005).
Kajiyama, H. et al. Neutral endopeptidase 24.11/CD10 suppresses progressive potential in ovarian carcinoma in vitro and in vivo. Clin. Cancer Res. 11, 1798–1808 (2005).
Meng, F. et al. Overexpression of membrane metalloendopeptidase inhibits substance P stimulation of cholangiocarcinoma growth. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G759–G768 (2014).
Stephen, H. M. et al. Epigenetic suppression of neprilysin regulates breast cancer invasion. Oncogenesis 5, e207 (2016).
Salles, G., Chen, C. Y., Reinherz, E. L. & Shipp, M. A. CD10/NEP is expressed on Thy-1low B220+ murine B-cell progenitors and functions to regulate stromal cell-dependent lymphopoiesis. Blood 80, 2021–2029 (1992).
Tarasoff-Conway, J. M. et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470 (2015).
Masters, C. L. & Selkoe, D. J. Biochemistry of amyloid ß-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a006262 (2012).
Morley, J. E. & Farr, S. A. The role of amyloid-beta in the regulation of memory. Biochem. Pharmacol. 88, 479–485 (2014).
Lambert, M. P. et al. Diffusible, nonfibrillar ligands derived from Aß1-42 are potent central nervous system neurotoxins. Proc. Natl Acad. Sci. USA 95, 6448–6453 (1998).
Dahlgren, K. N. et al. Oligomeric and fibrillar species of amyloid-ß peptides differentially affect neuronal viability. J. Biol. Chem. 277, 32046–32053 (2002).
Miners, J. S., Kehoe, P. & Love, S. Neprilysin protects against cerebral amyloid angiopathy and Aß-induced degeneration of cerebrovascular smooth muscle cells. Brain Pathol. 21, 594–605 (2011).
Fonseca, A. C. et al. Amyloid-beta disrupts calcium and redox homeostasis in brain endothelial cells. Mol. Neurobiol. 51, 610–622 (2015).
Wang, J., Dickson, D. W., Trojanowski, J. Q. & Lee, V. M. The levels of soluble versus insoluble brain Aß distinguish Alzheimer's disease from normal and pathologic aging. Exp. Neurol. 158, 328–337 (1999).
Herrup, K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 18, 794–799 (2015).
Musiek, E. S. & Holtzman, D. M. Three dimensions of the amyloid hypothesis: time, space and 'wingmen'. Nat. Neurosci. 18, 800–806 (2015).
Saido, T. & Leissring, M. A. Proteolytic degradation of amyloid ß-protein. Cold Spring Harb. Perspect. Med. 2, a006379 (2012).
Hafez, D. et al. Neprilysin-2 is an important ß-amyloid degrading enzyme. Am. J. Pathol. 178, 306–312 (2011).
Iwata, N., Higuchi, M. & Saido, T. C. Metabolism of amyloid-ß peptide and Alzheimer's disease. Pharmacol. Ther. 108, 129–148 (2005).
Kanemitsu, H., Tomiyama, T. & Mori, H. Human neprilysin is capable of degrading amyloid ß peptide not only in the monomeric form but also the pathological oligomeric form. Neurosci. Lett. 350, 113–116 (2003).
Farris, W. et al. Loss of neprilysin function promotes amyloid plaque formation and causes cerebral amyloid angiopathy. Am. J. Pathol. 171, 241–251 (2007).
Marr, R. A. et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J. Neurosci. 23, 1992–1996 (2003).
Parthasarathy, R. et al. Reduction of amyloid-beta levels in mouse eye tissues by intra-vitreally delivered neprilysin. Exp Eye Res. 138, 134–144 (2015).
El-Amouri, S. S. et al. Neprilysin: an enzyme candidate to slow the progression of Alzheimer's disease. Am. J. Pathol. 172, 1342–1354 (2008).
Spencer, B. et al. Long-term neprilysin gene transfer is associated with reduced levels of intracellular Abeta and behavioral improvement in APP transgenic mice. BMC Neurosci. 9, 109 (2008).
Guan, H. et al. Peripherally expressed neprilysin reduces brain amyloid burden: a novel approach for treating Alzheimer's disease. J. Neurosci. Res. 87, 1462–1473 (2009).
Spencer, B. et al. Peripheral delivery of a CNS targeted, metalo-protease reduces Aß toxicity in a mouse model of Alzheimer's disease. PLoS ONE 6, e16575 (2011).
Park, M. H. et al. Recombinant soluble neprilysin reduces amyloid-beta accumulation and improves memory impairment in Alzheimer's disease mice. Brain Res. 1529, 113–124 (2013).
Devi, L. & Ohno, M. A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice. Mol. Brain 8, 19 (2015).
Rose, J. B. et al. Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer's disease. J. Neurosci. 29, 1115–1125 (2009).
Iwata, N. et al. Identification of the major Aß1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat. Med. 6, 143–150 (2000).
Newell, A. J. et al. Thiorphan-induced neprilysin inhibition raises amyloid ß levels in rabbit cortex and cerebrospinal fluid. Neurosci. Lett. 350, 178–180 (2003).
Marr, R. A. et al. Neprilysin regulates amyloid ß peptide levels. J. Mol. Neurosci. 22, 5–11 (2004).
Eckman, E. A. et al. Regulation of steady-state ß-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J. Biol. Chem. 281, 30471–30478 (2006).
Mouri, A. et al. Inhibition of neprilysin by thiorphan (i.c.v.) causes an accumulation of amyloid ß and impairment of learning and memory. Behav. Brain Res. 168, 83–91 (2006).
Zou, L. B. et al. Inhibition of neprilysin by infusion of thiorphan into the hippocampus causes an accumulation of amyloid ß and impairment of learning and memory. J. Pharmacol. Exp. Ther. 317, 334–340 (2006).
Yasojima, K., Akiyama, H., McGeer, E. G. & McGeer, P. L. Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide. Neurosci. Lett. 297, 97–100 (2001).
Yasojima, K., McGeer, E. G. & McGeer, P. L. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 919, 115–121 (2001).
Wang, S. et al. Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer's brain. J. Neurochem. 115, 47–57 (2010).
Miners, J. S., van Helmond, Z., Kehoe, P. G. & Love, S. Changes with age in the activities of ß-secretase and the Aß-degrading enzymes neprilysin, insulin-degrading enzyme and angiotensin-converting enzyme. Brain Pathol. 20, 794–802 (2010).
Huang, J. Y., Hafez, D. M., James, B. D., Bennett, D. A. & Marr, R. A. Altered NEP2 expression and activity in mild cognitive impairment and Alzheimer's disease. J. Alzheimers Dis. 28, 433–441 (2012).
Maruyama, M. et al. Cerebrospinal fluid neprilysin is reduced in prodromal Alzheimer's disease. Ann. Neurol. 57, 832–842 (2005).
Sorensen, K. C., Simonsen, A. H., Holmetoft, U. B., Hasselbalch, S. G. & Heegaard, N. H. Neprilysin-like activity correlates with CSF-Tau and phospho-tau in patients with Alzheimer's disease. J. Alzheimers Dis. 37, 379–387 (2013).
Clarimon, J. et al. Possible increased risk for Alzheimer's disease associated with neprilysin gene. J. Neural Transm. (Vienna) 110, 651–657 (2003).
Helisalmi, S. et al. Polymorphisms in neprilysin gene affect the risk of Alzheimer's disease in Finnish patients. J. Neurol. Neurosurg. Psychiatry 75, 1746–1748 (2004).
Sakai, A. et al. Association of the Neprilysin gene with susceptibility to late-onset Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 17, 164–169 (2004).
Shi, J. et al. Mutation screening and association study of the neprilysin gene in sporadic Alzheimer's disease in Chinese persons. J. Gerontol. A Biol. Sci. Med. Sci. 60, 301–306 (2005).
Wood, L. S., Pickering, E. H., McHale, D. & Dechairo, B. M. Association between neprilysin polymorphisms and sporadic Alzheimer's disease. Neurosci. Lett. 427, 103–106 (2007).
Miners, S. et al. Genetic variation in MME in relation to neprilysin protein and enzyme activity, Aß levels, and Alzheimer's disease risk. Int. J. Mol. Epidemiol. Genet. 3, 30–38 (2012).
Wang, H. Z. et al. Neprilysin confers genetic susceptibility to Alzheimer's disease in Han Chinese. Mol. Neurobiol. 53, 4883–4892 (2016).
Sodeyama, N. et al. Lack of association of neprilysin polymorphism with Alzheimer's disease and Alzheimer's disease-type neuropathological changes. J. Neurol. Neurosurg. Psychiatry 71, 817–818 (2001).
Oda, M. et al. Dinucleotide repeat polymorphisms in the neprilysin gene are not associated with sporadic Alzheimer's disease. Neurosci. Lett. 320, 105–107 (2002).
Lilius, L. et al. No association between polymorphisms in the neprilysin promoter region and Swedish Alzheimer's disease patients. Neurosci. Lett. 337, 111–113 (2003).
Fu, Y. et al. Lack of association of neprilysin gene polymorphisms with Alzheimer's disease in a southern Chinese community. Int. Psychogeriatr. 21, 354–358 (2009).
Blomqvist, M. E., McCarthy, S., Blennow, K., Andersson, B. & Prince, J. A. Evaluation of neprilysin sequence variation in relation to CSF ß-amyloid levels and Alzheimer disease risk. Int. J. Mol. Epidemiol. Genet. 1, 47–52 (2010).
Debiec, H. et al. Role of truncating mutations in MME gene in fetomaternal alloimmunisation and antenatal glomerulopathies. Lancet 364, 1252–1259 (2004).
Koay, E. S. C. & Walmsley, N. A Primer of Chemical Pathology (World Scientific Publishing Co. Pte. Ltd., 1996).
Hajduk, A. M., Kiefe, C. I., Person, S. D., Gore, J. G. & Saczynski, J. S. Cognitive change in heart failure: a systematic review. Circ. Cardiovasc. Qual. Outcomes 6, 451–460 (2013).
Gehrs, K. M., Anderson, D. H., Johnson, L. V. & Hageman, G. S. Age-related macular degeneration — emerging pathogenetic and therapeutic concepts. Ann. Med. 38, 450–471 (2006).
Luibl, V. et al. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J. Clin. Invest. 116, 378–385 (2006).
Wang, J., Ohno-Matsui, K. & Morita, I. Elevated amyloid ß production in senescent retinal pigment epithelium, a possible mechanism of subretinal deposition of amyloid ß in age-related macular degeneration. Biochem. Biophys. Res. Commun. 423, 73–78 (2012).
Yoshida, T. et al. The potential role of amyloid ß in the pathogenesis of age-related macular degeneration. J. Clin. Invest. 115, 2793–2800 (2005).
Biffi, A. & Greenberg, S. M. Cerebral amyloid angiopathy: a systematic review. J. Clin. Neurol. 7, 1–9 (2011).
Attems, J., Lauda, F. & Jellinger, K. A. Unexpectedly low prevalence of intracerebral hemorrhages in sporadic cerebral amyloid angiopathy: an autopsy study. J. Neurol. 255, 70–76 (2008).
Carpentier, M., Robitaille, Y., DesGroseillers, L., Boileau, G. & Marcinkiewicz, M. Declining expression of neprilysin in Alzheimer disease vasculature: possible involvement in cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 61, 849–856 (2002).
Miners, J. S. et al. Decreased expression and activity of neprilysin in Alzheimer disease are associated with cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 65, 1012–1021 (2006).
Yamada, M. et al. Association of neprilysin polymorphism with cerebral amyloid angiopathy. J. Neurol. Neurosurg. Psychiatry 74, 749–751 (2003).
Tsubuki, S., Takaki, Y. & Saido, T. C. Dutch, Flemish, Italian, and Arctic mutations of APP and resistance of Aß to physiologically relevant proteolytic degradation. Lancet 361, 1957–1958 (2003).
Scholzen, T. E., Konig, S., Fastrich, M., Bohm, M. & Luger, T. A. Terminating the stress: peripheral peptidolysis of proopiomelanocortin-derived regulatory hormones by the dermal microvascular endothelial cell extracellular peptidases neprilysin and angiotensin-converting enzyme. Endocrinology 148, 2793–2805 (2007).
Lisy, O. et al. Neutral endopeptidase inhibition potentiates the natriuretic actions of adrenomedullin. Am. J. Physiol. Renal Physiol. 275, F410–F414 (1998).
Carson, J. A. & Turner, A. J. ß-Amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases? J. Neurochem. 81, 1–8 (2002).
Skidgel, R. A. & Erdos, E. G. Angiotensin converting enzyme (ACE) and neprilysin hydrolyze neuropeptides: a brief history, the beginning and follow-ups to early studies. Peptides 25, 521–525 (2004).
Allred, A. J., Diz, D. I., Ferrario, C. M. & Chappell, M. C. Pathways for angiotensin-(1–7) metabolism in pulmonary and renal tissues. Am. J. Physiol. Renal Physiol. 279, F841–F850 (2000).
Kenny, A. J., Bourne, A. & Ingram, J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem. J. 291, 83–88 (1993).
Katayama, M. et al. Catabolism of calcitonin gene-related peptide and substance P by neutral endopeptidase. Peptides 12, 563–567 (1991).
Sokolovsky, M. et al. Endothelins are more senstive than sarafotoxins to neutral endopeptidase: possible physiological significance. Proc. Natl Acad. Sci. USA 87, 4702–4706 (1990).
Hupe-Sodmann, K. et al. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7–36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul. Pept. 58, 149–156 (1995).
Trebbien, R. et al. Neutral endopeptidase 24.11 is important for the degradation of both endogenous and exogenous glucagon in anesthetized pigs. Am. J. Physiol. Endocrinol. Metab. 287, E431–E438 (2004).
Medeiros Mdos, S. & Turner, A. J. Metabolism and functions of neuropeptide Y. Neurochem. Res. 21, 1125–1132 (1996).
Johnson, A. R., Skidgel, R. A., Gafford, J. T. & Erdos, E. G. Enzymes in placental microvilli: angiotensin I converting enzyme, angiotensinase A, carboxypeptidase, and neutral endopeptidase (“enkephalinase”). Peptides 5, 789–796 (1984).
Medeiros, M. D. & Turner, A. J. Processing and metabolism of peptide-YY: pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology 134, 2088–2094 (1994).
Goetzl, E. J., Sreedharan, S. P., Turck, C. W., Bridenbaugh, R. & Malfroy, B. Preferential cleavage of amino- and carboxyl-terminal oligopeptides from vasoactive intestinal polypeptide by human recombinant enkephalinase (neutral endopeptidase, EC 3.4.24.11). Biochem. Biophys. Res. Commun. 158, 850–854 (1989).
Acknowledgements
St Vincent's Institute of Medical Research is supported in part by the Victorian Government's Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.J.C. has consulted for, and received financial and material support from, Novartis.
Rights and permissions
About this article
Cite this article
Campbell, D. Long-term neprilysin inhibition — implications for ARNIs. Nat Rev Cardiol 14, 171–186 (2017). https://doi.org/10.1038/nrcardio.2016.200
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2016.200
This article is cited by
-
Advancing Guideline-Directed Medical Therapy in Heart Failure: Overcoming Challenges and Maximizing Benefits
American Journal of Cardiovascular Drugs (2024)
-
Drugs in treating paediatric acute kidney injury
Pediatric Nephrology (2023)
-
Cooling down with Entresto. Can sacubitril/valsartan combination enhance browning more than coldness?
Diabetology & Metabolic Syndrome (2022)
-
Effect of sacubitril/valsartan and ACEI/ARB on glycaemia and the development of diabetes: a systematic review and meta-analysis of randomised controlled trials
BMC Medicine (2022)
-
Current and Emerging Classes of Pharmacological Agents for the Management of Hypertension
American Journal of Cardiovascular Drugs (2022)