Key Points
-
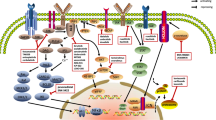
The availability of novel small-molecule inhibitors and immunotherapies has changed the landscape of drug development in lymphoma
-
The most effective small-molecule inhibitors target B-cell-receptor signalling, PI3K signalling, and the BCL2 protein
-
Multiple immunotherapy platforms have demonstrated promising clinical activity, including immune-checkpoint inhibitors, CAR T cells, and bispecific antibodies
-
Many companies are developing similar drugs, which inhibit the same target, necessitating a more focused drug development strategy, with prioritization of clinical investigations
-
Owing to the high number of drugs in development, the potential number of drug combinations is becoming unmanageable; prioritization should focus on mechanism-based combinations that are potentially safer and more effective
Abstract
The landscape of drugs for the treatment of lymphoma has become crowded in light of the plethora of new agents, necessitating the efficient prioritization of drugs for expedited development. The number of drugs available, and the fact that many can be given for an extended period of time, has resulted in the emergence of new challenges; these include determining the optimal duration of therapy, and the need to balance costs, benefits, and the risk of late-onset toxicities. Moreover, with the increase in the number of available investigational drugs, the number of possible combinations is becoming overwhelming, which necessitates prioritization plans for the selective development of novel combination regimens. In this Review, we describe the most-promising agents in clinical development for the treatment of lymphoma, and provide expert opinion on new strategies that might enable more streamlined drug development. We also address new approaches for patient selection and for incorporating new end points into clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
DeFrancesco, L. Drug pipeline Q4 2015. Nat. Biotechnol. 34, 128 (2016).
Wilson, W. H. et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 21, 922–926 (2015).
Friedberg, J. W. et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115, 2578–2585 (2010).
Wang, M. L. et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 369, 507–516 (2013).
Dreyling, M. et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 387, 770–778 (2016).
Byrd, J. C. et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 371, 213–223 (2014).
Treon, S. P. et al. Ibrutinib in previously treated Waldenstrom's macroglobulinemia. N. Engl. J. Med. 372, 1430–1440 (2015).
Younes, A. et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 15, 1019–1026 (2014).
Bartlett, N. L. et al. Ibrutinib monotherapy in relapsed/refractory follicular lymphoma (FL): preliminary results of a phase 2 consortium (P2C) trial. Blood 124, 800 (2014).
Christian, B. et al. A phase I study of ibrutinib and lenalidomide in patients with relapsed and refractory B-cell non-hodgkin's lymphoma. Blood 124, 4476 (2014).
Fowler, N. et al. Ibrutinib plus rituximab in treatment-naive patients with follicular lymphoma: results from a multicenter, phase 2 study. Blood 126, 470 (2015).
Ujjani, C. S. et al. Phase I study of rituximab, lenalidomide, and ibrutinib in previously untreated follicular lymphoma (alliance 051103). Blood 126, 471 (2015).
Woyach, J. A. et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 370, 2286–2294 (2014).
Cao, Y. et al. CXCR4 WHIM-like frameshift and nonsense mutations promote ibrutinib resistance but do not supplant MYD88L265P-directed survival signalling in Waldenstrom macroglobulinaemia cells. Br. J. Haematol. 168, 701–707 (2015).
Advani, R. H. et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 31, 88–94 (2013).
Mohamed, A. J. et al. Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev. 228, 58–73 (2009).
Readinger, J. A., Mueller, K. L., Venegas, A. M., Horai, R. & Schwartzberg, P. L. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol. Rev. 228, 93–114 (2009).
Andreotti, A. H., Schwartzberg, P. L., Joseph, R. E. & Berg, L. J. T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harb. Perspect. Biol. 2, a002287 (2010).
Dubovsky, J. A. et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 122, 2539–2549 (2013).
Byrd, J. C. et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 371, 323–332 (2015).
Tam, C. et al. The BTK inhibitor, Bgb-3111, is safe, tolerable, and highly active in patients with relapsed/ refractory B-cell malignancies: initial report of a phase 1 first-in-human trial. Blood 126, 832 (2015).
Gopal, A. K. et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 370, 1008–1018 (2014).
Coutré, S. E. et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk. Lymphoma 56, 2779–2786 (2015).
Khan, K. H. et al. Hyperglycemia and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) inhibitors in phase I trials: incidence, predictive factors, and management. Oncologist 21, 855–860 (2016).
Chiron, D. et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov. 4, 1022–1035 (2014).
Choudhary, G. S. et al. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 6, e1593 (2015).
Forero-Torres, A. et al. A phase 1 study of INCB040093, a PI3Kδ inhibitor, alone or in combination with INCB039110, a selective JAK1 inhibitor: interim results from patients (pts) with relapsed or refractory (r/r) classical Hodgkin lymphoma (cHL). ASCO Meeting Abstracts 33 (Suppl. 15), 8558 (2015).
Gaudio, E. et al. Combination of the MEK inhibitor pimasertib with BTK or PI3K-δ inhibitors is active in preclinical models of aggressive lymphomas. Ann. Oncol. 27, 1123–1128 (2016).
Lee, J. S., Tang, S. S., Ortiz, V., Vo, T. T. & Fruman, D. A. MCL-1-independent mechanisms of synergy between dual PI3K/mTOR and BCL-2 inhibition in diffuse large B cell lymphoma. Oncotarget 6, 35202–35217 (2015).
Younes, A. et al. Safety, tolerability, and preliminary activity of CUDC-907, a first-in-class, oral, dual inhibitor of HDAC and PI3K, in patients with relapsed or refractory lymphoma or multiple myeloma: an open-label, dose-escalation, phase 1 trial. Lancet Oncol. 17, 622–631 (2016).
Cheah, C. Y. et al. Lenalidomide, idelalisib, and rituximab are unacceptably toxic in patients with relapsed/refractory indolent lymphoma. Blood 125, 3357–3359 (2015).
Green, D. R. A BH3 mimetic for killing cancer cells. Cell 165, 1560 (2016).
Davids, M. S. & Letai, A. ABT-199: taking dead aim at BCL-2. Cancer Cell 23, 139–141 (2013).
Roberts, A. W. et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. Br. J. Haematol. 170, 669–678 (2015).
Wilson, W. H. et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 11, 1149–1159 (2010).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013).
Roberts, A. W. et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2015).
Jones, J. et al. Preliminary results of a phase 2, open-label study of venetoclax (ABT-199/GDC-0199) monotherapy in patients with chronic lymphocytic leukemia relapsed after or refractory to ibrutinib or idelalisib therapy. Blood 126, 715 (2015).
Gerecitano, J. F. et al. A phase 1 study of venetoclax (ABT-199 / GDC-0199) monotherapy in patients with relapsed/refractory non-Hodgkin lymphoma. Blood 126, 254 (2015)
Phillips, D. C. et al. Loss in MCL-1 function sensitizes non-Hodgkin's lymphoma cell lines to the BCL-2-selective inhibitor venetoclax (ABT-199). Blood Cancer J. 5, e368 (2015).
Oppermann, S. et al. Identification of kinase inhibitors that overcome venetoclax resistance in activated CLL cells by high-content screening. Blood 128, 934–947 (2016).
Derenzini, E. & Younes, A. Targeting the JAK-STAT pathway in lymphoma: a focus on pacritinib. Expert Opin. Investig. Drugs 22, 775–785 (2013).
O'Shea, J. J. et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 66, 311–328 (2015).
Younes, A. et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J. Clin. Oncol. 30, 4161–4167 (2012).
Blunt, M. D. et al. The Syk\\Jak inhibitor cerdulatinib shows promising preclinical activity in chronic lymphocytic leukemia by antagonising B cell receptor and microenvironmental signalling. Blood 126, 1716 (2015).
Leeds, J. M. et al. Abstract CT144: preclinical and clinical studies and modeling and simulation to identify phase II dose for cerdulatinib: a dual SYK/JAK inhibitor for the treatment of B-cell malignancies. Cancer Res. 76 (Suppl 14), CT144 (2016).
Gribben, J. G., Fowler, N. & Morschhauser, F. Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J. Clin. Oncol. 33, 2803–2811 (2015).
Witzig, T. E. et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. J. Clin. Oncol. 27, 5404–5409 (2009).
Vose, J. M. et al. Single-agent lenalidomide is active in patients with relapsed or refractory aggressive non-Hodgkin lymphoma who received prior stem cell transplantation. Br. J. Haematol. 162, 639–647 (2013).
Witzig, T. E. et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann. Oncol. 22, 1622–1627 (2011).
Goy, A. et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J. Clin. Oncol. 31, 3688–3695 (2013).
Fowler, N. H. et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 15, 1311–1318 (2014).
Wang, M. et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 13, 716–723 (2012).
Ruan, J. et al. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N. Engl. J. Med. 373, 1835–1844 (2015).
Nowakowski, G. S. et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non–germinal center B-cell phenotype in newly diagnosed diffuse large B-cell lymphoma: a phase II study. J. Clin. Oncol. 33, 251–257 (2015).
Vitolo, U. et al. Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol. 15, 730–737 (2014).
Batlevi, C. L., Matsuki, E., Brentjens, R. J. & Younes, A. Novel immunotherapies in lymphoid malignancies. Nat. Rev. Clin. Oncol. 13, 25–40 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Campbell, M. T., Siefker-Radtke, A. O. & Gao, J. The state of immune checkpoint inhibition in urothelial carcinoma: current evidence and future areas of exploration. Cancer J. 22, 96–100 (2016).
Naidoo, J., Page, D. B. & Wolchok, J. D. Immune checkpoint blockade. Hematol. Oncol. Clin. North Am. 28, 585–600 (2014).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Ansell, S. M. et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 15, 6446–6453 (2009).
Armand, P. et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J. Clin. Oncol. 31, 4199–4206 (2013).
Westin, J. R. et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 15, 69–77 (2014).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Armand, P. et al. PD-1 Blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: safety, efficacy, and biomarker assessment. Blood 126, 584 (2015).
Zinzani, P. L. et al. Phase 1b study of PD-1 blockade with pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL). Blood 126, 3986 (2015).
Younes, A. et al. Checkmate 205: Nivolumab (nivo) in classical Hodgkin lymphoma (cHL) after autologous stem cell transplant (ASCT) and brentuximab vedotin (BV)—A phase 2 study. ASCO Meeting Abstracts 34 (Suppl 15), 7535 (2016).
Lesokhin, A. M. et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J. Clin. Oncol. 34, 2698–2704 (2016).
Till, B. G. et al. Safety and clinical activity of atezolizumab (anti-PDL1) in combination with obinutuzumab in patients with relapsed or refractory non-Hodgkin lymphoma. Blood 126, 5104 (2015).
Sagiv-Barfi, I. et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl. Acad. Sci. USA 112, E966–E972 (2015).
Kochenderfer, J. N. et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33, 540–549 (2015).
Ramos, C. A., Heslop, H. E. & Brenner, M. K. CAR-T cell therapy for lymphoma. Annu. Rev. Med. 67, 165–183 (2016).
Sadelain, M. CAR therapy: the CD19 paradigm. J. Clin. Invest. 125, 3392–3400 (2015).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra138 (2013).
Schuster, S. J. et al. Sustained remissions following chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. Blood 126, 183 (2015).
Ryan, M. et al. SGN-CD198, a pyrrolobenzodiazepine (PBD)-based anti-CD19 drug conjugate, demonstrates potent preclinical activity against B-cell malignacies. Blood 126, (2015).
McCombs, J. R. & Owen, S. C. Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J. 17, 339–351 (2015).
Younes, A. et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 363, 1812–1821 (2010).
Advani, A. et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study. J. Clin. Oncol. 28, 2085–2093 (2010).
Advani, R. et al. A phase I study of DCDT2980S, an antibody-drug conjugate (ADC) targeting CD22, in relapsed or refractory B-cell non-Hodgkin's lymphoma (NHL). Abs 59. Blood 120, 59 (2012).
Ribrag, V. et al. A dose-escalation study of SAR3419, an anti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 20, 213–220 (2014).
Palanca-Wessels, M. C. et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 16, 704–715 (2015).
Bartlett, N. et al. A phase 2 study of Brentuximab Vedotin in patients with relapsed or refractory CD30-positive non-Hodgkin lymphomas: interim results in patients with DLBCL and other B-cell lymphomas. Blood 122, 848 (2013).
Bartlett, N. L. et al. Brentuximab vedotin monotherapy in DLBCL patients with undetectable CD30: preliminary results from a phase 2 study. Blood 124, 629 (2014).
Forero-Torres, A. et al. Interim analysis of a phase 1, open-label, dose-escalation study of SGN-CD19A in patients with relapsed or refractory B-lineage non-Hodgkin lymphoma (NHL). J. Clin. Oncol. 32 (Suppl 5), 8505 (2014).
Morschhauser, F. et al. Preliminary results of a phase II randomized study (ROMULUS) of polatuzumab vedotin (PoV) or pinatuzumab vedotin (PiV) plus rituximab (RTX) in pts with relapsed/refractory non-Hodgkin lymphoma. J. Clin. Oncol. 32 (Suppl 5), 8519 (2014).
Jung, M., Gelato, K. A., Fernandez-Montalvan, A., Siegel, S. & Haendler, B. Targeting BET bromodomains for cancer treatment. Epigenomics 7, 487–501 (2015).
Delmore, J. E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011).
Boi, M. et al. The BET bromodomain inhibitor OTX015 affects pathogenetic pathways in preclinical B-cell tumor models and synergizes with targeted drugs. Clin. Cancer Res. 21, 1628–1638 (2015).
Amorim, S. et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 3, e196–204 (2016).
Abramson, J. S. et al. BET inhibitor CPI-0610 is well tolerated and induces responses in diffuse large B-cell lymphoma and follicular lymphoma: preliminary analysis of an ongoing phase 1 study. Blood 126, 1491 (2015).
Morin, R. D. et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat. Genet. 42, 181–185 (2010).
Beguelin, W. et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 23, 677–692 (2013).
Velichutina, I. et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood 116, 5247–5255 (2010).
Qi, W. et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc. Natl. Acad. Sci. USA 109, 21360–21365 (2012).
McCabe, M. T. et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492, 108–112 (2012).
Ribrag, V. et al. Phase 1 study of tazemetostat (EPZ-6438), an inhibitor of enhancer of zeste-homolog 2 (EZH2): Preliminary safety and activity in relapsed or refractory non-Hodgkin lymphoma (NHL) patients. Blood 126, 473 (2015).
Younes, A. Beyond chemotherapy: new agents for targeted treatment of lymphoma. Nat. Rev. Clin. Oncol. 8, 85–96 (2011).
Intlekofer, A. M. & Younes, A. Precision therapy for lymphoma—current state and future directions. Nat. Rev. Clin. Oncol. 11, 585–596 (2014).
Armand, P. et al. Detection of circulating tumour DNA in patients with aggressive B-cell non-Hodgkin lymphoma. Br. J. Haematol. 163, 123–126 (2013).
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224ra224 (2014).
Diehl, F. et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 14, 985–990 (2008).
Faham, M. et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood 120, 5173–5180 (2012).
Arnold, A. et al. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N. Engl. J. Med. 309, 1593–1599 (1983).
Roschewski, M. et al. Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: a correlative biomarker study. Lancet Oncol. 16, 541–549 (2015).
Author information
Authors and Affiliations
Contributions
All authors researched data for article, contributed to discussion of the content, wrote the manuscript and reviewed/edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
A.Y. receives honouraria and/or consults for Abbvie, Bayer, BMS, Celgene, Gilead, Incyte, Janssen R&D, Sanofi, Seattle Genetics, Merck, and Takeda Millenium, and research support from the John and Barbara Vogelstein Foundation. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Younes, A., Ansell, S., Fowler, N. et al. The landscape of new drugs in lymphoma. Nat Rev Clin Oncol 14, 335–346 (2017). https://doi.org/10.1038/nrclinonc.2016.205
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.205
This article is cited by
-
The novel LSD1 inhibitor ZY0511 suppresses diffuse large B-cell lymphoma proliferation by inducing apoptosis and autophagy
Medical Oncology (2021)
-
LncRNA HCP5 promotes LAML progression via PSMB8-mediated PI3K/AKT pathway activation
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)
-
DuoHexaBody-CD37®, a novel biparatopic CD37 antibody with enhanced Fc-mediated hexamerization as a potential therapy for B-cell malignancies
Blood Cancer Journal (2020)
-
The Role of PI3K Inhibition in Lymphoid Malignancies
Current Hematologic Malignancy Reports (2019)
-
Dual effect of DLBCL-derived EXOs in lymphoma to improve DC vaccine efficacy in vitro while favor tumorgenesis in vivo
Journal of Experimental & Clinical Cancer Research (2018)