Key Points
-
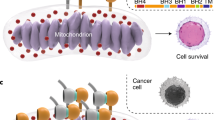
The intrinsic pathway to apoptotic cell death converges on events at the mitochondrial outer membrane where interactions between the extended BCL-2 family of proteins determine the fate of the cell. The detailed mechanisms by which pro-survival and anti-survival factions of the family perform their roles remain under investigation.
-
There is now compelling evidence that cell death can be triggered by agents that mimic the action of a helical peptide in binding to the pro-survival family members. The target for these putative cytotoxic agents, an extended groove on the surface of the pro-survival BCL-2 family proteins, is a protein–protein interaction site, presenting particular challenges to drug discovery.
-
Cytotoxic activity does not necessarily require direct antagonism of the BCL-2 family, presenting challenges to establishing the mode of action.
-
More than a dozen candidate chemical classes have been claimed to antagonize one or more of the members of the pro-survival BCL-2 protein family. Most of these discoveries have come from screening compound libraries of one type or another. Design work has been limited to constrained helical peptides, terphenyls and benzoylureas, but only the helical peptides have interesting potency. Among the screening hits, many seem to exert their cytotoxicity through a non-apoptotic pathway.
-
Compounds that have progressed into clinical trials include GX15-070 (GeminX), AT-101 (Ascenta) and ABT-263 (Abbott). For only one of these three compound classes (ABT-263) is structural data currently available to guide further refinement of binding affinity and pharmacological properties.
-
GX15-070 (IC50 of the order of 1 μM for its BCL-2 targets) is effective as a single agent in killing multiple cancer cell lines in vitro and in vivo, and is administered intravenously in clinical trials against various haematological malignancies. This compound has also been shown to cause cell-cycle arrest, at concentrations lower than those that induce apoptosis. Further evaluation of its mechanism of action is needed.
-
AT-101 (IC50 of the order of 200 nM for its BCL-2 targets), the (–) enantiomer of gossypol, has similar in vitro cytotoxicity to GX15-070 and is administered orally in clinical studies for treatment of chronic lymphocytic leukaemia. Its mechanism of action is called into question by reports that gossypol kills cells lacking the apoptotic killers BAK and BAX.
-
ABT-263 (IC50 of the order of 1 nM for its BCL-2 targets), an orally available analogue of ABT-737, is insensitive to cells lacking BAK and BAX. It shows single-agent activity against various haematological tumours and small-cell lung carcinomas that have low levels of the pro-survival family member MCL1, to which ABT-263 shows no appreciable binding.
-
The mechanism of action of a bona fide BCL-2 family antagonist should be established by four criteria. One, cell-killing must be BAK/BAX dependent. Two, binding affinity for the relevant BCL-2 family targets should be in the low nanomolar range, similar to their physiological ligands. Three, the binding profile of the antagonist to the five pro-survival BCL-2 family members should correlate with cellular activity and expression levels of these proteins. Four, treatment of animals with the antagonist should trigger relevant biomarkers that correlate with the binding profile of the drug and the known biological roles of the five pro-survival family members.
Abstract
Overexpression of members of the BCL-2 family of pro-survival proteins is commonly associated with unfavourable pathogenesis in cancer. The convergence of cytotoxic stress signals on the extended BCL-2 protein family provides the biological rationale for directly targeting this family to induce apoptotic cell death. Recently, several compounds have been described that inhibit the interaction between BCL-2 family members and their natural ligand, a helical peptide sequence known as the BH3 domain. Here, we review preclinical and clinical data on these compounds, and recommend four criteria that define antagonists of the BCL-2 protein family.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vaux, D. L., Cory, S. & Adams, J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442 (1988). The discovery of the pro-survival function of BCL-2.
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Adams, J. M. & Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26, 1324–1337 (2007).
Youle, R. J. & Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Rev. Mol. Cell Biol. 9, 47–59 (2008).
Kvansakul, M. et al. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 15, 1564–1571 (2008).
Huang, D. C. & Strasser, A. BH3-only proteins — essential initiators of apoptotic cell death. Cell 103, 839–842 (2000).
Muchmore, S. W. et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381, 335–341 (1996). The BCL-2 fold is revealed, together with the locations of the BH domains.
Sattler, M. et al. Structure of Bcl-xL–Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983–986 (1997). The BH3 domain of a pro-apoptotic protein is seen to bind in a groove on a pro-survival protein, establishing the paradigm for interactions between family members.
Willis, S. N. et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315, 856–859 (2007).
Certo, M. et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 (2006).
Kuwana, T. et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17, 525–535 (2005).
Lindsten, T. et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399 (2000).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001).
Shimizu, S. et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nature Cell Biol. 6, 1221–1228 (2004).
Oberstein, A., Jeffrey, P. D. & Shi, Y. G. Crystal structure of the Bcl-X-L–beclin 1 peptide complex — Beclin 1 is a novel BH3-only protein. J. Biol. Chem. 282, 13123–13132 (2007).
Maiuri, M. C. et al. Functional and physical interaction between Bcl-X-L and a BH3-like domain in Beclin-1. EMBO J. 26, 2527–2539 (2007).
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Levine, B. Cell biology — autophagy and cancer. Nature 446, 745–747 (2007).
Motoyama, N. et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267, 1506–1510 (1995).
Veis, D. J., Sorenson, C. M., Shutter, J. R. & Korsmeyer, S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75, 229–240 (1993).
Rinkenberger, J. L., Horning, S., Klocke, B., Roth, K. & Korsmeyer, S. J. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 14, 23–27 (2000).
Ross, A. J. et al. Testicular degeneration in Bclw-deficient mice. Nature Genet. 18, 251–6 (1998).
Mason, K. D. et al. Programmed anuclear cell death delimits platelet life span. Cell 128, 1173–1186 (2007).
Chen, L. et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393–403 (2005). Some of the BH3-only proteins bind promiscuously to pro-survival proteins, others selectively. Broad neutralization of pro-survival family members is needed for effective cell death.
Oda, E. et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 (2000).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Strasser, A. The role of BH3-only proteins in the immune system. Nature Rev. Immunol. 5, 189–200 (2005).
Puthalakath, H. et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349 (2007).
Fesik, S. W. Promoting apoptosis as a strategy for cancer drug discovery. Nature Rev. Cancer 5, 876–885 (2005).
Warr, M. R. & Shore, G. C. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr. Mol. Med. 8, 138–147 (2008).
Derenne, S. et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or BCI-xL is an essential survival protein of human myeloma cells. Blood 100, 194–199 (2002).
Alvi, A. J. et al. A novel CDK inhibitor, CYC202 (R-roscovitine), overcomes the defect in p53-dependent apoptosis in B-CLL by down-regulation of genes involved in transcription regulation and survival. Blood 105, 4484–4491 (2005).
Amundson, S. A. et al. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 60, 6101–6110 (2000).
Willis, S. N. et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19, 1294–1305 (2005).
Lee, E. F. et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J. Cell Biol. 180, 341–355 (2008).
Petros, A. M. et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 9, 2528–2534 (2000).
Walensky, L. D. et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305, 1466–1470 (2004).
Walensky, L. D. et al. A stapled BID BH3 helix directly binds and activates BAX. Mol. Cell 24, 199–210 (2006).
Sadowsky, J. D. et al. (α/β+α)-peptide antagonists of BH3 domain/Bcl-xL recognition: toward general strategies for foldamer-based inhibition of protein–protein interactions. J. Am. Chem. Soc. 129, 139–154 (2007).
Kutzki, O. et al. Development of a potent Bcl-xL antagonist based on α-helix mimicry. J. Am. Chem. Soc. 124 11838–11839 (2002).
Davis, J. M., Truong, A. & Hamilton, A. D. Synthesis of a 2,3′;6′,3′′-terpyridine scaffold as an α-helix mimetic. Org. Lett. 7, 5405–5408 (2005).
Yin, H. & Hamilton, A. D. Terephthalamide derivatives as mimetics of the helical region of Bak peptide target Bcl-xL protein. Bioorg. Med. Chem. Lett. 14, 1375–1379 (2004).
Lessene, G. L. & Baell, J. B. Alpha-helical mimetics. US2008153802 (2008).
Degterev, A. et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nature Cell Biol. 3, 173–182 (2001).
Wang, J. L. et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl Acad. Sci. USA 97, 7124–7129 (2000).
Enyedy, I. J. et al. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J. Med. Chem. 44, 4313–4324 (2001).
Tzung, S. P. et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nature Cell Biol. 3, 183–191 (2001).
Chan, S.-L. et al. Identification of chelerythrine as an inhibitor of BclXL function. J. Biol. Chem. 278, 20453–20456 (2003).
Kitada, S. et al. Discovery, characterization, and structure–activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J. Med. Chem. 46, 4259–4264 (2003).
Leone, M. et al. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 63, 8118–8121 (2003).
van Delft, M. F. et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10, 389–399 (2006). Unlike ABT-737, many of the putative BCL-2 antagonists are sensitive on cells containing neither BAK nor BAX, suggesting that they do not kill by apoptosis.
Baell, J. B. & Huang, D. C. Prospects for targeting the Bcl-2 family of proteins to develop novel cytotoxuc drugs. Biochem. Pharmacol. 64, 851–863 (2002).
Crawford, N. & Fennell, D. A. Small molecule de-repression of BAX and BAK oligomerization as a strategy for treating cancer. Lett. Drug Design Discov. 3, 534–540 (2006).
Murthy, M. S. et al. A small molecule inhibitor of BCL-2 protein–protein interactions specifically induces apoptosis in cancer cells. Clin. Cancer Res. 7, 3717S (2001).
Perez-Galan, P., Roue, G., Villamor, N., Campo, E. & Colomer, D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood 109, 4441–4449 (2007).
Zhai, D., Jin, C., Satterthwait, A. C. & Reed, J. C. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 13, 1419–1421 (2006).
Reed, J. C. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 13, 1378–1386 (2006).
Nguyen, M. et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc. Natl Acad Sci. USA 104, 19512–19517 (2007). Molecular and cellular data supporting a role for GX15-070 in antagonizing MCL1.
Li, J., Viallet, J. & Haura, E. B. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother. Pharmacol. 61, 525–534 (2008).
Dong, S. et al. Targeting 14-3-3 sensitizes native and mutant BCR–ABL to inhibition with U0126, rapamycin and Bcl-2 inhibitor GX15-070 Leukemia 22, 572–577 (2008).
Witters, L. M. et al. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol. Rep. 17, 465–469 (2007).
Konopleva, M. et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res. 68, 3413–3420 (2008). GX15-070 causes cell-cycle arrest at concentrations 30-times lower than the IC 50 for induction of apoptosis.
Bebb, D., Muzik, H., Nguen, S., Chen, S. & Morris, D. GX15-070 enhances the anti-lymphoma effect of vinorelbine in a murine model of mantle cell lymphoma. Ann. Oncol. 18, 34 (2007).
Schimmer, A. D. et al. A phase I trial of the small molecule Pan-Bcl-2 family inhibitor obatoclax mesylate (GX15-070) administered by continuous infusion for up to four days to patients with hematological malignancies. Blood 110, 272A (2007).
Borthakur, G. et al. Phase I trial of the small molecule Pan-Bcl-2 family inhibitor obatoclax mesylate (GX15-070) administered by 24 hour infusion every 2 weeks to patients with myeloid malignancies and, chronic lymphocytic leukemia (CLL). Blood 108, 750A (2006).
Verstovsek, S., Raza, A., Schimmer, A. D., Viallet, J. & Kantarjian, H. A phase II trial of the small molecule pan-Bcl-2 family inhibitor obatoclax mesylate (GX15-070) administered by a 24-h continuous infusion every 2 weeks to patients with chronic idiopathic myelofibrosis (CIMF). Blood 110, 1040A (2007).
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008). Preclinical data for ABT-263 provide grounds for clinical evaluation against small-cell lung cancer and B-cell malignancies.
Dodou, K., Anderson, R. J., Small, D. A. & Groundwater, P. W. Investigations on gossypol: past and present developments. Expert Opin. Investig. Drugs 14, 1419–1434 (2005).
Qiu, J. P., Levin, L. R., Buck, J. & Reidenberg, M. M. Different pathways of cell killing by gossypol enantiomers. Exp. Biol. Med. 227, 398–401 (2002).
Tang, G. Z. et al. Acylpyrogallols as inhibitors of antiapoptotic Bcl-2 proteins. J. Med. Chem. 51, 717–720 (2008).
Paoluzzi, L. et al. Targeting antiapoptotic Bcl-2 family members with AT-101 in pre-clinical models of aggressive lymphoma in combination with cyclophosphamide (C) and rituximab (R) produces a marked improvement in therapeutic efficacy. Blood 106, 680A–681A (2005).
Kumar, S. et al. AT-101, a small molecule inhibitor of Bcl-2 family proteins, has significant in vitro activity in multiple myeloma. Blood 106, 453A–454A (2005).
James, D. F., Prada, C. E., Castro, J. E. & Kipps, T. J. AT 101, an inhibitor of Bcl-2 family members is cytotoxic to a heterogeneous group of CLL samples and synergistic with rituximab. Blood 106, 835A (2005).
James, D. F. et al. AT-101, a small molecule Bcl-2 antagonist, in treatment naive CLL patients (pts) with high risk features; preliminary results from an ongoing phase I trial. J. Clin. Oncol. 24, 362S (2006).
Castro, J. E. et al. A phase II, open label study of AT-101 in combination with rituximab in patients with relapsed or refractory chronic lymphocytic leukemia. Evaluation of two dose regimens. Blood 110, 917A–918A (2007).
Castro, J. E. et al. A phase II, open label study of AT-101 in combination with rituximab in patients with relapsed or refractory chronic lymphocytic leukemia. Blood 108, 803A (2006).
Lei, X. B. et al. Gossypol induces Bax/Bak-independent activation of apoptosis and cytochrome c release via a conformational change in Bcl-2. FASEB J. 20, 2147–2149 (2006).
Wang, G. P. et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J. Med. Chem. 49, 6139–6142 (2006).
Tang, G. Z. et al. Structure-based design of flavonoid compounds as a new class of small-molecule inhibitors of the anti-apoptotic bcl-2 proteins. J. Med. Chem. 50, 3163–3166 (2007).
Verhaegen, M. et al. A novel BH3 mimetic reveals a mitogen-activated protein kinase-dependent mechanism of melanoma cell death controlled by p53 and reactive oxygen species. Cancer Res. 66, 11348–11359 (2006).
Zeitlin, B. D. et al. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res. 66, 8698–8706 (2006).
Arnold, A. A. et al. Preclinical studies of apogossypolone: a new nonpeptidic pan small-molecule inhibitor of Bcl-2, Bcl-X-L and Mcl-1 proteins in follicular small cleaved cell lymphoma model. Mol. Cancer 7, 20–30 (2008).
Schreiber, S. L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287, 1964–1969 (2000).
Castro, C. C. et al. Compounds and methods for inhibiting the interaction of bcl proteins with binding partners WO2008060569 (2008).
Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005). The discovery of ABT-737 from fragment screening and structure-guided design to improve potency and pharmacological properties. Efficacy against solid tumours in animal models is demonstrated.
Petros, A. M. et al. Discovery of a potent inhibitor of the antiapoptotic protein Bcl-xL from NMR and parallel synthesis. J. Med. Chem. 49, 656–663 (2006).
Wendt, M. D. et al. Discovery and structure–activity relationship of antagonists of B-cell lymphoma 2 family proteins with chemopotentiation activity in vitro and in vivo. J. Med. Chem. 49, 1165–1181 (2006).
Shoemaker, A. R. et al. A small-molecule inhibitor of Bcl-X-L potentiates the activity of cytotoxic drugs in vitro and in vivo. Cancer Res. 66, 8731–8739 (2006).
Bruncko, M. et al. Studies leading to potent, dual inhibitors of Bcl-2 and Bcl-xL. J. Med. Chem. 50, 641–662 (2007).
Lee, E. F. et al. Crystal structure of ABT-737 complexed with Bcl-xL: implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 14, 1711–1713 (2007). Description of the structure of the complex between ABT-737 and BCL-X L , and a rationale for its failure to bind MCL1.
Veber, D. F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 (2002).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997).
Vaux, D. L. ABT-737, proving to be a great tool even before it is proven in the clinic. Cell Death Differ. 15, 807–808 (2008).
Chen, S., Dai, Y., Harada, H., Dent, P. & Grant, S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 67, 782–791 (2007).
Konopleva, M. et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10, 375–388 (2006).
Tahir, S. K. et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 67, 1176–1183 (2007).
Lin, X. et al. 'Seed' analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-X-L inhibitor ABT-737. Oncogene 26, 3972–3979 (2007).
Vogler, M. et al. A novel paradigm for rapid ABT-737-induced apoptosis involving outer mitochondrial membrane rupture in primary leukemia and lymphoma cells. Cell Death Differ. 15, 820–830 (2008).
Kline, M. P. et al. ABT-737, an inhibitor of Bcl-2 family proteins, is a potent inducer of apoptosis in multiple myeloma cells. Leukemia 21, 1549–1560 (2007).
Lock, R. et al. Initial testing (stage 1) of the BH3 mimetic ABT-263 by the pediatric preclinical testing program. Pediatr. Blood Cancer 50, 1181–1189 (2008).
Shoemaker, A. R. et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin. Cancer Res. 14, 3268–3277 (2008).
Mason, K. D. et al. The delicate balance between pro survival BCL XL and pro apoptotic BAK determines platelet life span. Exp. Hematol. 35 (Suppl. 2), 33–33 (2007).
Zhang, H. et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 14, 943–951 (2007).
Labi, V., Erlacher, M., Kiessling, S. & Villunger, A. BH3-only proteins in cell death initiation, malignant disease and anticancer therapy. Cell Death Differ. 13, 1325–1338 (2006).
Kuroda, J. et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc. Natl Acad. Sci. USA 103, 14907–14912 (2006).
Li, R., Moudgil, T., Ross, H. J. & Hu, H. M. Apoptosis of non-small-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ. 12, 292–303 (2005).
Lee, E. F. et al. EGL-1 BH3 mutants reveal the importance of protein levels and target affinity for cell-killing potency. Cell Death Differ. 15, 1609–1618 (2008).
Yin, H. & Hamilton, A. D. Strategies for targeting protein–protein interactions with synthetic agents. Angewandte Chemie Int. Ed. 44, 4130–4163 (2005).
Colman, P. M. in Structure-Based Drug Discovery — An Overview (ed. Hubbard, R. E.) 193–218 (The Royal Society of Chemistry Publishing, Cambridge, 2006).
Hinds, M. G. et al. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 14, 128–136 (2007).
Chou, J. J., Li, H., Salvesen, G. S., Yuan, J. & Wagner, G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96, 615–624 (1999).
Hinds, M. G. & Day, C. L. Regulation of apoptosis: uncovering the binding determinants. Curr. Opin. Struct. Biol. 15, 690–699 (2005).
Petros, A. M., Olejniczak, E. T. & Fesik, S. W. Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 1644, 83–94 (2004).
Smits, C., Czabotar, P. E., Hinds, M. G. & Day, C. L. Structural plasticity underpins promiscuous binding of the prosurvival protein A1. Structure 16, 818–829 (2008).
Czabotar, P. E. et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc. Natl Acad. Sci. USA 104, 6217–6222 (2007).
Liu, X., Dai, S., Zhu, Y., Marrack, P. & Kappler, J. The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity 19, 341–352 (2003).
Day, C. L. et al. Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J. Biol. Chem. 280, 4738–4744 (2005).
Berman, H. M. et al. The Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
Acknowledgements
We acknowledge helpful discussions with D. Huang and many other colleagues at The Walter and Eliza Hall Institute of Medical Research including J. Adams, J. Baell, S. Cory, D. Fairlie, E. Lee and K. Watson. Work in the authors' laboratories is supported by the National Health and Medical Research Council (Australia), The Leukemia and Lymphoma Society (USA), The Cancer Council of Victoria and The Australian Cancer Research Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The Walter and Eliza Hall Institute of Medical Research has an ongoing research collaboration agreement with Genentech and Abbott in the field of apoptosis, specifically BCL-2 family proteins.
Related links
Glossary
- Mitochondrial pathway to programmed cell death
-
This pathway is activated by intracellular stresses and developmental cues. Also known as the intrinsic or stress pathway, it is regulated by the extended BCL-2 protein family. By contrast, the extrinsic apoptotic pathway is activated by cell-surface death receptors.
- Caspase cascade
-
A proteolytic cascade that is initiated by both the mitochondrial pathway and the extrinsic pathway.
- Autophagy
-
A cellular stress response in which non-vital cell components are broken down to provide nutrients for vital processes. Autophagy may also serve as an alternative non-apoptotic mechanism for cell death under certain conditions.
- Lymphopaenia
-
A condition marked by lower than normal circulating lymphocytes.
- Thrombocytopaenia
-
A decrease in platelet count, defined in humans as less than 150 million per ml, which results in the potential for increased bleeding.
- Endoplasmic reticulum stress
-
Cellular disturbances causing an accumulation of unfolded proteins in the endoplasmic reticulum, which results in activation of the unfolded protein response.
- Metathesis reaction
-
A transition metal-catalysed reaction used to manipulate alkenes.
Rights and permissions
About this article
Cite this article
Lessene, G., Czabotar, P. & Colman, P. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov 7, 989–1000 (2008). https://doi.org/10.1038/nrd2658
Issue Date:
DOI: https://doi.org/10.1038/nrd2658
This article is cited by
-
CYD0281, a Bcl-2 BH4 domain antagonist, inhibits tumor angiogenesis and breast cancer tumor growth
BMC Cancer (2023)
-
The Musashi RNA-binding proteins in female cancers: insights on molecular mechanisms and therapeutic relevance
Biomarker Research (2023)
-
BCL-W makes only minor contributions to MYC-driven lymphoma development
Oncogene (2023)
-
Identification of bicyclic compounds that act as dual inhibitors of Bcl-2 and Mcl-1
Molecular Diversity (2023)
-
Constitutively Synergistic Multiagent Drug Formulations Targeting MERTK, FLT3, and BCL-2 for Treatment of AML
Pharmaceutical Research (2023)