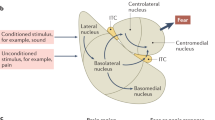
Abstract
Post-traumatic stress disorder (PTSD) occurs in 5–10% of the population and is twice as common in women as in men. Although trauma exposure is the precipitating event for PTSD to develop, biological and psychosocial risk factors are increasingly viewed as predictors of symptom onset, severity and chronicity. PTSD affects multiple biological systems, such as brain circuitry and neurochemistry, and cellular, immune, endocrine and metabolic function. Treatment approaches involve a combination of medications and psychotherapy, with psychotherapy overall showing greatest efficacy. Studies of PTSD pathophysiology initially focused on the psychophysiology and neurobiology of stress responses, and the acquisition and the extinction of fear memories. However, increasing emphasis is being placed on identifying factors that explain individual differences in responses to trauma and promotion of resilience, such as genetic and social factors, brain developmental processes, cumulative biological and psychological effects of early childhood and other stressful lifetime events. The field of PTSD is currently challenged by fluctuations in diagnostic criteria, which have implications for epidemiological, biological, genetic and treatment studies. However, the advent of new biological methodologies offers the possibility of large-scale approaches to heterogeneous and genetically complex brain disorders, and provides optimism that individualized approaches to diagnosis and treatment will be discovered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: (DSM-5) (American Psychiatric Association, 2013).
Kessler, R. C. & Wang, P. S. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu. Rev. Publ. Health 29, 115–129 (2008).
Michopoulos, V. et al. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry 172, 353–362 (2015).
Lohr, J. B. et al. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. Am. J. Geriatr. Psychiatry 23, 709–725 (2015).
Rosenbaum, S. et al. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism 64, 926–933 (2015).
Kessler R. C. Posttraumatic stress disorder: the burden to the individual and to society. J. Clin. Psychiatry 61 S4–S12; discussion S13–S14 (2000).
Yehuda, R. & LeDoux, J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56, 19–32 (2007).
Charney, D. S., Deutch, A. Y., Krystal, J. H., Southwick, S. M. & Davis, M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch. General Psychiatry 50, 295–305 (1993).
Charney, D. S. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am. J. Psychiatry 161, 195–216 (2004).
Kulka, R. A. Contractual Report of Findings From the National Vietnam Veterans Readjustment Study (Research Triangle Institute, 1988).
Dohrenwend, B. P. et al. The psychological risks of Vietnam for US veterans: a revisit with new data and methods. Science 313, 979–982 (2006).
Marmar, C. R. et al. Course of posttraumatic stress disorder 40 years after the Vietnam War: findings from the National Vietnam Veterans Longitudinal Study. JAMA Psychiatry 72, 875–881 (2015). This is the most important longitudinal study of the Vietnam War generation.
Kok, B. C., Herrell, R. K., Thomas, J. L. & Hoge, C. W. Posttraumatic stress disorder associated with combat service in Iraq or Afghanistan: reconciling prevalence differences between studies. J. Nerv. Mental Dis. 200, 444–450 (2012).
Sundin, J. et al. Mental health outcomes in US and UK military personnel returning from Iraq. Br. J. Psychiatry 204, 200–207 (2014).
Hoge, C. W. et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N. Engl. J. Med. 351, 13–22 (2004). This is the initial paper that showed the effect of the recent war in Iraq and highlighted the importance of stigma and barriers to help-seeking.
Smid, G. E., Mooren, T. T., van der Mast, R. C., Gersons, B. P. & Kleber, R. J. Delayed posttraumatic stress disorder: systematic review, meta-analysis, and meta-regression analysis of prospective studies. J. Clin. Psychiatry 70, 1572–1582 (2009).
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M. & Nelson, C. B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. General Psychiatry 52, 1048–1060 (1995). Results from this study indicate that trauma exposure is common but only a minority of exposed individuals develop PTSD. Findings also showed that conditional PTSD varied by trauma type and that the disorder is highly comorbid with other psychiatric conditions.
Resnick, H. S., Kilpatrick, D. G., Dansky, B. S., Saunders, B. E. & Best, C. L. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J. Consult. Clin. Psychol. 61, 984 (1993).
American Psychiatric Association. DSM-III: Diagnostic and Statistical Manual of Mental Disorders, Third Edition (American Psychiatric Association, 1985).
Mills, K. L. et al. Assessing the prevalence of trauma exposure in epidemiological surveys. Austral. N. Z. J. Psychiatry 45, 407–415 (2011).
De Jong, J. T. et al. Lifetime events and posttraumatic stress disorder in 4 postconflict settings. JAMA 286, 555–562 (2001).
Atwoli, L. et al. Trauma and posttraumatic stress disorder in South Africa: analysis from the South African Stress and Health Study. BMC Psychiatry 13, 182 (2013).
Karam, E. G. et al. Cumulative traumas and risk thresholds: 12-month PTSD in the World Mental Health (WMH) surveys. Depress. Anxiety 31, 130–142 (2014).
Hobfoll, S. E. et al. Five essential elements of immediate and mid-term mass trauma intervention: empirical evidence. Psychiatry 70, 283–315; discussion 316–369 (2007).
Lowe, S. R., Galea, S., Uddin, M. & Koenen, K. C. Trajectories of posttraumatic stress among urban residents. Am. J. Commun. Psychol. 53, 159–172 (2014).
Ressler, K. J. et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470, 492–497 (2011).
Kessler, R. C., Chiu, W. T., Demler, O. & Walters, E. E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. General Psychiatry 62, 617–627 (2005).
Hoge, C. W., Terhakopian, A., Castro, C. A., Messer, S. C. & Engel, C. C. Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq war veterans. Am. J. Psychiatry 164, 150–153 (2007).
Pole, N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol. Bull. 133, 725–746 (2007).
Pitman, R. K. et al. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787 (2012).
Zoladz, P. R. & Diamond, D. M. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neurosci. Biobehav. Rev. 37, 860–895 (2013).
Yehuda, R. Post-traumatic stress disorder. N. Engl. J. Med. 346, 108–114 (2002).
Zannas, A. S., Provencal, N. & Binder, E. B. Epigenetics of posttraumatic stress disorder: current evidence, challenges, and future directions. Biol. Psychiatry 78, 327–335 (2015).
Daskalakis, N. P., Lehrner, A. & Yehuda, R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol. Metab. Clin. North Am. 42, 503–513 (2013).
Galatzer-Levy, I. R. et al. Cortisol response to an experimental stress paradigm prospectively predicts long-term distress and resilience trajectories in response to active police service. J. Psychiatr. Res. 56, 36–42 (2014).
van Zuiden, M. et al. Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. Am. J. Psychiatry 168, 89–96 (2011). This is the first biological prospective study in PTSD that showed that combat veterans who developed PTSD post-deployment had a higher number of glucocorticoid receptors at pre-deployment.
de Quervain, D. J., Aerni, A., Schelling, G. & Roozendaal, B. Glucocorticoids and the regulation of memory in health and disease. Front. Neuroendocrinol. 30, 358–370 (2009).
Parsons, R. G. & Ressler, K. J. Implications of memory modulation for post-traumatic stress and fear disorders. Nat. Neurosci. 16, 146–153 (2013).
Duits, P. et al. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress. Anxiety 32, 239–253 (2015).
Jovanovic, T. & Ressler, K. J. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry 167, 648–662 (2010).
Pietrzak, R. H. et al. Association of in vivo κ-opioid receptor availability and the transdiagnostic dimensional expression of trauma-related psychopathology. JAMA Psychiatry 71, 1262–1270 (2014).
Neumeister, A., Seidel, J., Ragen, B. J. & Pietrzak, R. H. Translational evidence for a role of endocannabinoids in the etiology and treatment of posttraumatic stress disorder. Psychoneuroendocrinology 51, 577–584 (2015).
O'Donovan, A., Slavich, G. M., Epel, E. S. & Neylan, T. C. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci. Biobehav Rev. 37, 96–108 (2013). This is a comprehensive review of the relationship of threat sensitivity and inflammation to increased risk for diseases of ageing in PTSD.
Baker, D. G. et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 9, 209–217 (2001).
Bonne, O. et al. Corticotropin-releasing factor, interleukin-6, brain-derived neurotrophic factor, insulin-like growth factor-1, and substance P in the cerebrospinal fluid of civilians with posttraumatic stress disorder before and after treatment with paroxetine. J. Clin. Psychiatry 72, 1124–1128 (2011).
Uddin, M. et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc. Natl Acad. Sci. USA 107, 9470–9475 (2010).
Gill, J. M., Saligan, L., Woods, S. & Page, G. PTSD is associated with an excess of inflammatory immune activities. Perspect. Psychiatr. Care 45, 262–277 (2009).
O'Donovan, A. et al. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis. Markers 30, 123–132 (2011).
Hill, M. N. & McEwen, B. S. Endocannabinoids: the silent partner of glucocorticoids in the synapse. Proc. Natl Acad. Sci. USA 106, 4579–4580 (2009).
Hill, M. N. et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 38, 2952–2961 (2013).
Neumeister, A. et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol. Psychiatry 18, 1034–1040 (2013).
Neylan, T. C., Otte, C., Yehuda, R. & Marmar, C. R. Neuroendocrine regulation of sleep disturbances in PTSD. Ann. NY Acad. Sci. 1071, 203–215 (2006).
Agorastos, A., Kellner, M., Baker, D. G. & Otte, C. When time stands still: an integrative review on the role of chronodisruption in posttraumatic stress disorder. Curr. Opin. Psychiatry 27, 385–392 (2014).
Yehuda, R., Teicher, M. H., Trestman, R. L., Levengood, R. A. & Siever, L. J. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol. Psychiatry 40, 79–88 (1996).
Cohen, H. et al. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 37, 350–363 (2012).
Serova, L. I. et al. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience 236, 298–312 (2013).
Yehuda, R., Flory, J. D., Southwick, S. & Charney, D. S. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann. NY Acad. Sci. 1071, 379–396 (2006).
Vermetten, E., Baker, D. & Yehuda, R. New findings from prospective studies. Psychoneuroendocrinology 51, 441–443 (2015).
Delahanty, D. L. & Nugent, N. R. Predicting PTSD prospectively based on prior trauma history and immediate biological responses. Ann. NY Acad. Sci. 1071, 27–40 (2006).
Bonne, O. et al. Prospective evaluation of plasma cortisol in recent trauma survivors with posttraumatic stress disorder. Psychiatry Res. 119, 171–175 (2003).
Yehuda, R. et al. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am. J. Psychiatry 171, 872–880 (2014).
Yehuda, R., McFarlane, A. C. & Shalev, A. Y. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol. Psychiatry 44, 1305–1313 (1998).
Yehuda, R., Morris, A., Labinsky, E., Zemelman, S. & Schmeidler, J. Ten-year follow-up study of cortisol levels in aging holocaust survivors with and without PTSD. J. Trauma Stress 20, 757–761 (2007).
Olff, M., de Vries, G. J., Guzelcan, Y., Assies, J. & Gersons, B. P. Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinology 32, 619–626 (2007).
Walsh, K. et al. Cortisol at the emergency room rape visit as a predictor of PTSD and depression symptoms over time. Psychoneuroendocrinology 38, 2520–2528 (2013).
Delahanty, D. L., Raimonde, A. J. & Spoonster, E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol. Psychiatry 48, 940–947 (2000).
van Zuiden, M. et al. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol. Psychiatry 71, 309–316 (2012).
Eraly, S. A. et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry 71, 423–431 (2014).
Yehuda, R. et al. Glucocorticoid-related predictors and correlates of post-traumatic stress disorder treatment response in combat veterans. Interface Focus 4, 20140048 (2014).
Rauch, S. A. et al. Biological and symptom changes in posttraumatic stress disorder treatment: a randomized clinical trial. Depress. Anxiety 32, 204–212 (2015).
Levy-Gigi, E., Szabo, C., Kelemen, O. & Keri, S. Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol. Psychiatry 74, 793–800 (2013).
Afifi, T. O., Asmundson, G. J., Taylor, S. & Jang, K. L. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin. Psychol. Rev. 30, 101–112 (2010).
Stein, M. B., Jang, K. J., Taylor, S., Vernon, P. A. & Livesley, W. J. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder: a twin study. Am. J. Psychiatry 159, 1675–1681 (2002).
Koenen, K. C., Duncan, L. E., Liberzon, I. & Ressler, K. J. From candidate genes to genome-wide association: the challenges and promise of posttraumatic stress disorder genetic studies. Biol. Psychiatry 74, 634–636 (2013).
True, W. J. et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch. General Psychiatry 50, 257–264 (1993).
Sartor, C. E. et al. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch. General Psychiatry 69, 293–299 (2012).
Sartor, C. E. et al. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol. Med. 41, 1497–1505 (2011).
Gilbertson, M. W. et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 5, 1242–1247 (2002).
Wolf, E. J., Mitchell, K. S., Koenen, K. C. & Miller, M. W. Combat exposure severity as a moderator of genetic and environmental liability to post-traumatic stress disorder. Psychol. Med. 44, 1499–1509 (2013).
Binder, E. B. et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299, 1291–1305 (2008). This paper describes that genetic variants of a chaperone protein involved in glucocorticoid signalling, together with exposure to child trauma, predict adult PTSD.
Klengel, T. et al. Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nat. Neurosci. 16, 33–41 (2013).
Nugent, N. R., Amstadter, A. B. & Koenen, K. C. Genetics of post-traumatic stress disorder: informing clinical conceptualizations and promoting future research. Am. J. Med. Genet. C Semin. Med. Genet. 148, 127–132 (2008).
Yehuda, R., Bell, A., Bierer, L. M. & Schmeidler, J. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. J. Psychiatr. Res. 42, 1104–1111 (2008).
Mehta, D. et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc. Natl Acad. Sci. USA 110, 8302–8307 (2013).
Almli, L. M. et al. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces. Am. J. Med. Genet. B Neuropsychiatr. Genet. 168, 327–336 (2015).
Heijmans, B. T. & Mill, J. Commentary: the seven plagues of epigenetic epidemiology. Int. J. Epidemiol. 41, 74–78 (2012).
Yehuda, R. et al. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front. Psychiatry 4, 118 (2013).
Yehuda, R. et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol. Psychiatry 66, 708–711 (2009). This is the first genome-wide gene expression study in PTSD that identified genes associated with glucocorticoid receptor functioning, such as FKBP5.
Sipahi, L. et al. Longitudinal epigenetic variation of DNA methyltransferase genes is associated with vulnerability to post-traumatic stress disorder. Psychol. Med. 44, 3165–3179 (2014).
Schmidt, U., Keck, M. E. & Buell, D. R. miRNAs and other non-coding RNAs in posttraumatic stress disorder: a systematic review of clinical and animal studies. J. Psychiatr. Res. 65, 1–8 (2015).
Zhou, J. et al. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS ONE 9, e94075 (2014).
Logue, M. W. et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor-α (RORA) gene as a significant risk locus. Mol. Psychiatry 18, 937–942 (2013).
Xie, P. et al. Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder. Biol. Psychiatry 74, 656–663 (2013).
Guffanti, G. et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology 38, 3029–3038 (2013).
Nievergelt, C. M. et al. Genomic predictors of combat stress vulnerability and resilience in U. S. Marines: a genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology 51, 459–471 (2015).
Button, K. S. et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013).
Almli, L. M., Fani, N., Smith, A. K. & Ressler, K. J. Genetic approaches to understanding post-traumatic stress disorder. Int. J. Neuropsychopharmacol. 17, 355–370 (2014).
Logue, M. W. et al. The Psychiatric Genomics Consortium Posttraumatic Stress Disorder Workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology 40, 2287–2297 (2015). This paper describes the formation of a large-scale GWAS consortium dedicated to the study of PTSD genetics that will lead the search for replicable genetic associations.
Bremner, J. D. Does stress damage the brain? Biol. Psychiatry 45, 797–805 (1999).
Bremner, J. D. et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse — a preliminary report. Biol. Psychiatry 41, 23–32 (1997).
van Rooij, S. J. et al. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol. Med. 45, 2737–2746 (2015).
Shin, L. M. & Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191 (2010).
Shin, L. M. et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am. J. Psychiatry 156, 575–584 (1999).
Gold, A. L. et al. Decreased regional cerebral blood flow in medial prefrontal cortex during trauma-unrelated stressful imagery in Vietnam veterans with post-traumatic stress disorder. Psychol. Med. 41, 2563–2572 (2011).
Lanius, R. A. et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol. Psychiatry 53, 204–210 (2003).
Etkin, A. & Wager, T. D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488 (2007).
Sartory, G. et al. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD). PLoS ONE 8, e58150 (2013).
Taber, K. H., Rauch, S. L., Lanius, R. A. & Hurley, R. A. Functional magnetic resonance imaging: application to posttraumatic stress disorder. J. Neuropsychiatry Clin. Neurosci. 15, 125–129 (2003).
Hayes, J. P., Hayes, S. M. & Mikedis, A. M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2, 9 (2012).
Lanius, R. A., Bluhm, R., Lanius, U. & Pain, C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J. Psychiatr. Res. 40, 709–729 (2006).
Nicholson, A. A. et al. The dissociative subtype of posttraumatic stress disorder: unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology 40, 2317–2326 (2015).
Lanius, R. et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am. J. Psychiatry 167, 640–647 (2010). This article focuses on the neural manifestations of the dissociative subtype in PTSD and compares them to those underlying the re-experiencing and hyperaroused subtype. These findings have important implications for the treatment of PTSD, including the need to assess patients with PTSD for dissociative symptoms and to incorporate the treatment of dissociative symptoms into stage-oriented trauma treatment.
Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506 (2011).
Patel, R., Spreng, R. N., Shin, L. M. & Girard, T. A. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 36, 2130–2142 (2012).
Sripada, R. K. et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomat. Med. 74, 904–911 (2012).
Rabellino, D. et al. Intrinsic connectivity networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatr. Scand. 35, 258–266 (2015).
Daniels, J. K. et al. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J. Psychiatry Neurosci. 35, 258–266 (2010).
Geuze, E. et al. Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol. Psychiatry 13, 74–83, 3 (2008).
Murrough, J. W. et al. Reduced amygdala serotonin transporter binding in posttraumatic stress disorder. Biol. Psychiatry 70, 1033–1038 (2011).
Yehuda, R. et al. Changes in relative glucose metabolic rate following cortisol administration in aging veterans with posttraumatic stress disorder: an FDG-PET neuroimaging study. J. Neuropsychiatry Clin. Neurosci. 21, 132–143 (2009).
Brewin, C. R. Episodic memory, perceptual memory, and their interaction: foundations for a theory of posttraumatic stress disorder. Psychol. Bull. 140, 69–97 (2014).
Yehuda, R. & McFarlane, A. C. Conflict between current knowledge about posttraumatic stress disorder and its original conceptual basis. Am. J. Psychiatry 152, 1705–1713 (1995). This is a seminal review showing that biological measures from PTSD do not conform to predictions from known acute stress biology.
American Psychiatric Association. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994).
Friedman, M. J., Resick, P. A., Bryant, R. A. & Brewin, C. R. Considering PTSD for DSM-5. Depress. Anxiety 28, 750–769 (2011).
Hoge, C. W., Riviere, L. A., Wilk, J. E., Herrell, R. K. & Weathers, F. W. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry 1, 269–277 (2014).
McFarlane, A. C. PTSD and DSM-5: unintended consequences of change. Lancet Psychiatry 1, 246–247 (2014).
Stein, D. J. et al. DSM-5 and ICD-11 definitions of posttraumatic stress disorder: investigating ‘narrow’ and ‘broad’ approaches. Depress. Anxiety 31, 494–505 (2014). This paper highlights the conceptual questions of validity with the existence of different sets of diagnostic criteria and the need to consider the challenge of the lack of overlap of DSM and ICD systems.
Bryant, R. A., O'Donnell, M. L., Creamer, M., McFarlane, A. C. & Silove, D. A multisite analysis of the fluctuating course of posttraumatic stress disorder. JAMA Psychiatry 70, 839–846 (2013).
Bunting, B. P., Murphy, S. D., O'Neill, S. M. & Ferry, F. R. Lifetime prevalence of mental health disorders and delay in treatment following initial onset: evidence from the Northern Ireland Study of Health and Stress. Psychol. Med. 42, 1727–1739 (2012).
McFarlane, A. C. The long-term costs of traumatic stress: intertwined physical and psychological consequences. World Psychiatry 9, 3–10 (2010). This paper shows that the prevalence of delayed-onset PTSD and the associated physical comorbidities highlight the need for the long-term effect of traumatic stress exposure to be a clear focus in clinical settings.
Kendler, K. S., Thornton, L. M. & Gardner, C. O. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the ‘kindling’ hypothesis. Am. J. Psychiatry 157, 1243–1251 (2000).
Collip, D., Myin-Germeys, I. & Van Os, J. Does the concept of ‘sensitization’ provide a plausible mechanism for the putative link between the environment and schizophrenia? Schizophr. Bull. 34, 220–225 (2008).
Adler, A. B., Wright, K. M., Bliese, P. D., Eckford, R. & Hoge, C. W. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J. Trauma. Stress 21, 301–308 (2008).
Brady, K. T., Killeen, T. K., Brewerton, T. & Lucerini, S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J. Clin. Psychiatry 61 S22–S32 (2000).
Debell, F. et al. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc. Psychiatry Psychiatr. Epidemiol. 49, 1401–1425 (2014).
Rytwinski, N. K., Scur, M. D., Feeny, N. C. & Youngstrom, E. A. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J. Trauma. Stress 26, 299–309 (2013).
Rasmussen, A., Keatley, E. & Joscelyne, A. Posttraumatic stress in emergency settings outside North America and Europe: a review of the emic literature. Soc. Sci. Med. 109, 44–54 (2014).
Brewin, C. R. et al. Promoting mental health following the London bombings: a screen and treat approach. J. Trauma. Stress 21, 3–8 (2008). This paper shows the underestimation of the clinical significance of distress and symptoms by general practitioners when diagnosing PTSD, despite patients seeking help and benefiting from treatment when identified in a population-based screening and treatment intervention.
Gupta, M. A. Review of somatic symptoms in post-traumatic stress disorder. Int. Rev. Psychiatry 25, 86–99 (2013).
Liebschutz, J. et al. PTSD in urban primary care: high prevalence and low physician recognition. J. Gen. Intern. Med. 22, 719–726 (2007).
Battaglia, S. et al. Elevated NCOR1 disrupts PPARα/γ signaling in prostate cancer and forms a targetable epigenetic lesion. Carcinogenesis 31, 1650–1660 (2010).
Lehrner, A. & Yehuda, R. Biomarkers of PTSD: military applications and considerations. Eur. J. Psychotraumatol. 5, 23797 (2014).
Searle, A. K. et al. The validity of military screening for mental health problems: diagnostic accuracy of the PCL, K10 and AUDIT scales in an entire military population. Int. J. Methods Psychiatr. Res. 24, 32–45 (2015).
Lee, D. J., Warner, C. H. & Hoge, C. W. Advances and controversies in military posttraumatic stress disorder screening. Curr. Psychiatry Rep. 16, 467 (2014).
Terhakopian, A., Sinaii, N., Engel, C. C., Schnurr, P. P. & Hoge, C. W. Estimating population prevalence of posttraumatic stress disorder: an example using the PTSD checklist. J. Trauma Stress 21, 290–300 (2008).
Australian Centre for Posttraumatic Mental Health. Australian Guidelines for the Treatment of Acute Stress Disorder and Posttraumatic Stress Disorder (ACPMH, 2013).
Warner, C. H., Appenzeller, G. N., Parker, J. R., Warner, C. M. & Hoge, C. W. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am. J. Psychiatry 168, 378–385 (2011).
Institute of Medicine. Preventing Psychological Disorders in Service Members and Their Families: An Assessment of Programs (The National Academies Press, 2014).
McCabe, O. L. et al. Building a national model of public mental health preparedness and community resilience: validation of a dual-intervention, systems-based approach. Disaster Med. Publ. Health Prep. 8, 511–526 (2014).
Yehuda, R. Learning from September 11, 2001. CNS Spectr. 7, 566–567 (2002).
Roberts, N. P., Kitchiner, N. J., Kenardy, J. & Bisson, J. Multiple session early psychological interventions for the prevention of post-traumatic stress disorder. Cochrane Database Syst. Rev. 3, CD006869 (2009).
Vermetten, E., Zhohar, J. & Krugers, H. J. Pharmacotherapy in the aftermath of trauma; opportunities in the ‘golden hours’. Curr. Psychiatry Rep. 16, 455 (2014).
Sijbrandij, M., Kleiboer, A., Bisson, J. I., Barbui, C. & Cuijpers, P. Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Lancet Psychiatry 2, 413–421 (2015). This is the first systematic review to examine the effects of pharmacotherapies (for example, β-blockers, hydrocortisone and selective serotonin reuptake inhibitors) given within the first month after a traumatic or aversive event to prevent PTSD or acute stress disorder compared with no pharmacotherapy or a placebo control.
Marin, M. F., Lonak, S. F. & Milad, M. R. Augmentation of evidence-based psychotherapy for PTSD with cognitive enhancers. Curr. Psychiatry Rep. 17, 39 (2015).
Merlo, E., Milton, A. L. & Everitt, B. J. Enhancing cognition by affecting memory reconsolidation. Curr. Opin. Behav. Sci. 4, 41–47 (2015).
Saxe, G. et al. Relationship between acute morphine and the course of PTSD in children with burns. J. Am. Acad. Child Adolesc. Psychiatry 40, 915–921 (2001).
Bryant, R. A., Creamer, M., O'Donnell, M., Silove, D. & McFarlane, A. C. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol. Psychiatry 65, 438–440 (2009).
Pitman, R. K. et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol. Psychiatry 51, 189–192 (2002).
Amos, T., Stein, D. J. & Ipser, J. C. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD). Cochrane Database Syst. Rev. 7, CD006239 (2014). This Cochrane Database Systematic Review focuses on the effects of pharmacological interventions for the prevention of adult PTSD. Evidence suggests that hydrocortisone might be moderately effective for the prevention of PTSD. There was no evidence to support the use of propranolol, temazapam, gabapentin and escitalopram in the prevention of PTSD onset.
McFarlane, A. C., Barton, C. A., Yehuda, R. & Wittert, G. Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology 36, 720–727 (2011).
Mouthaan, J. et al. The role of acute cortisol and DHEAS in predicting acute and chronic PTSD symptoms. Psychoneuroendocrinology 45, 179–186 (2014).
Schelling, G. et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol. Psychiatry 50, 978–985 (2001).
Zohar, J. et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur. Neuropsychopharmacol. 21, 796–809 (2011).
Delahanty, D. L. et al. The efficacy of initial hydrocortisone administration at preventing posttraumatic distress in adult trauma patients: a randomized trial. CNS Spectr. 18, 103–111 (2013).
Holbrook, T. L., Galarneau, M. R., Dye, J. L., Quinn, K. & Dougherty, A. L. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N. Engl. J. Med. 362, 110–117 (2010).
Markowitz, J. C. et al. Is exposure necessary? A randomized clinical trial of interpersonal psychotherapy for PTSD. Am. J. Psychiatry 172, 430–440 (2015).
Bisson, J. & Andrew, M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst. Rev. 3, CD003388 (2007). This Cochrane Database Systematic Review reports the effects of randomized controlled trials of individual trauma-focused treatments for adult PTSD. Results indicate that individual trauma-focused CBT and EMDR were superior to wait-list and usual care in reducing clinician-assessed PTSD symptoms.
Bisson, J. I., Roberts, N. P., Andrew, M., Cooper, R. & Lewis, C. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst. Rev. 12, CD003388 (2013).
Mello, P. G., Silva, G. R., Donat, J. C. & Kristensen, C. H. An update on the efficacy of cognitive-behavioral therapy, cognitive therapy, and exposure therapy for posttraumatic stress disorder. Int. J. Psychiatry Med. 46, 339–357 (2013).
Rauch, S. A., Eftekhari, A. & Ruzek, J. I. Review of exposure therapy: a gold standard for PTSD treatment. J. Rehabil. Res. Dev. 49, 679–687 (2012).
McLean, C. P. & Foa, E. B. Prolonged exposure therapy for post-traumatic stress disorder: a review of evidence and dissemination. Expert Rev. Neurother. 11, 1151–1163 (2011).
Morkved, N. et al. A comparison of narrative exposure therapy and prolonged exposure therapy for PTSD. Clin. Psychol. Rev. 34, 453–467 (2014).
Leer, A., Engelhard, I. M., Altink, A. & van den Hout, M. A. Eye movements during recall of aversive memory decreases conditioned fear. Behav. Res. Ther. 51, 633–640 (2013).
Engelhard, I. M. et al. Reducing vividness and emotional intensity of recurrent ‘flashforwards’ by taxing working memory: an analogue study. J. Anxiety Disord. 25, 599–603 (2011).
Seidler, G. H. & Wagner, F. E. Comparing the efficacy of EMDR and trauma-focused cognitive-behavioral therapy in the treatment of PTSD: a meta-analytic study. Psychol. Med. 36, 1515–1522 (2006).
Schottenbauer, M. A., Glass, C. R., Arnkoff, D. B. & Gray, S. H. Contributions of psychodynamic approaches to treatment of PTSD and trauma: a review of the empirical treatment and psychopathology literature. Psychiatry 71, 13–34 (2008).
Ehlers, A. et al. Predicting response to exposure treatment in PTSD: the role of mental defeat and alienation. J. Trauma Stress 11, 457–471 (1998).
Schnurr, P. P. et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA 297, 820–830 (2007).
Stein, D. J., Ipser, J. & McAnda, N. Pharmacotherapy of posttraumatic stress disorder: a review of meta-analyses and treatment guidelines. CNS Spectr. 14, 25–31 (2009).
Eisenman, D. et al. The ISTSS/Rand guidelines on mental health training of primary healthcare providers for trauma-exposed populations in conflict-affected countries. J. Trauma Stress 19, 5–17 (2006).
Weine, S. et al. Guidelines for international training in mental health and psychosocial interventions for trauma exposed populations in clinical and community settings. Psychiatry 65, 156–164 (2002).
Cloitre, M. et al. Treatment of complex PTSD: results of the ISTSS expert clinician survey on best practices. J. Trauma Stress 24, 615–627 (2011).
Rosen, C. S. et al. VA practice patterns and practice guidelines for treating posttraumatic stress disorder. J. Trauma Stress 17, 213–222 (2004).
Susskind, O., Ruzek, J. I. & Friedman, M. J. The VA/DOD clinical practice guideline for management of post-traumatic stress (update 2010): development and methodology. J. Rehabil. Res. Dev. 4, xvii–xxviii (2012).
Wolf, E. J., Lunney, C. A. & Schnurr, P. P. The influence of the dissociative subtype of posttraumatic stress disorder on treatment efficacy in female veterans and active duty service members. J. Consult Clin. Psychol. http://dx.doi.org/10.1037/ccp0000036 (2015).
Cloitre, M. et al. Treatment for PTSD related to childhood abuse: a randomized controlled trial. Am. J. Psychiatry 167, 915–924 (2010).
Cloitre, M., Petkova, E., Wang, J. & Lu Lassell, F. An examination of the influence of a sequential treatment on the course and impact of dissociation among women with PTSD related to childhood abuse. Depress. Anxiety 29, 709–717 (2012).
Lanius, R. A., Frewen, P. A., Tursich, M., Jetly, R. & McKinnon, M. C. Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. Eur. J. Psychotraumatol. 6, 27313 (2015).
Dorahy, M. J. et al. Dissociation, shame, complex PTSD, child maltreatment and intimate relationship self-concept in dissociative disorder, chronic PTSD and mixed psychiatric groups. J. Affect. Disord. 172, 195–203 (2014).
Albucher, R. C. & Liberzon, I. Psychopharmacological treatment in PTSD: a critical review. J. Psychiatr. Res. 36, 355–367 (2002).
Schoenfeld, F. B., Marmar, C. R. & Neylan, T. C. Current concepts in pharmacotherapy for posttraumatic stress disorder. Psychiatr. Serv. 55, 519–531 (2004).
Drici, M. D. & Priori, S. Cardiovascular risks of atypical antipsychotic drug treatment. Pharmacoepidemiol. Drug Saf. 16, 882–890 (2007).
Hageman, I., Andersen, H. S. & Jorgensen, M. B. Post-traumatic stress disorder: a review of psychobiology and pharmacotherapy. Acta Psychiatr. Scand. 104, 411–422 (2001).
Watanabe, Y., Sakai, R. R., McEwen, B. S. & Mendelson, S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 615, 87–94 (1993).
Yehuda, R. et al. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: relation to risk and resilience factors. J. Psychiatr. Res. 41, 435–445 (2007).
Neylan, T. C. et al. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol. Psychiatry 68, 494–496 (2010).
Karl, A. et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev. 30, 1004–1031 (2006).
Raskind, M. A. et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am. J. Psychiatry 170, 1003–1010 (2013).
Department of Veterans Affairs; VA Puget Sound Health Care System. Prazosin and combat trauma PTSD (PACT). ClinicalTrials.gov[online], (2014).
de Quervain, D. J. Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD. Prog. Brain Res. 167, 239–247 (2008).
Golier, J. A., Caramanica, K., Demaria, R. & Yehuda, R. A pilot study of mifepristone in combat-related PTSD. Depress Res. Treat. 2012, 393251 (2012).
Aerni, A. et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am. J. Psychiatry 161, 1488–1490 (2004).
Suris, A., North, C., Adinoff, B., Powell, C. M. & Greene, R. Effects of exogenous glucocorticoid on combat-related PTSD symptoms. Ann. Clin. Psychiatry 22, 274–279 (2010).
Ipser, J. C. & Stein, D. J. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD). Int. J. Neuropsychopharmacol. 15, 825–840 (2012).
Management of Post-Traumatic Stress Working Group. VA/DoD clinical practice guideline for the management of post-traumatic stress. U.S. Department of Veteran Affairs[online], (2010).
Dunlop, B. W. et al. Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: study protocol for a randomized controlled trial. Trials 15, 240 (2014).
Cameron, C., Watson, D. & Robinson, J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J. Clin. Psychopharmacol. 34, 559–564 (2014).
Jetly, R., Heber, A., Fraser, G. & Boisvert, D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 51, 585–588 (2015).
Feder, A. et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 71, 681–688 (2014).
de Kleine, R. A., Rothbaum, B. O. & van Minnen, A. Pharmacological enhancement of exposure-based treatment in PTSD: a qualitative review. Eur. J. Psychotraumatol. 4, 21626 (2013).
Kupferschmidt, K. Can ecstasy treat the agony of PTSD? Science 345, 22–23 (2014).
Novakovic, V. et al. Brain stimulation in posttraumatic stress disorder. Eur. J. Psychotraumatol. 2, 5609 (2011).
Karsen, E. F., Watts, B. V. & Holtzheimer, P. E. Review of the effectiveness of transcranial magnetic stimulation for post-traumatic stress disorder. Brain Stimul. 7, 151–157 (2014).
Steckler, T. & Risbrough, V. Pharmacological treatment of PTSD — established and new approaches. Neuropharmacology 62, 617–627 (2012).
Rothbaum, B. O. et al. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am. J. Psychiatry 171, 640–648 (2014).
Yehuda, R. et al. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology 51, 589–597 (2015).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (American Psychiatric Association, 2000).
Pietrzak, R. H. et al. Functional significance of a novel 7-factor model of DSM-5 PTSD symptoms: results from the National Health and Resilience in Veterans Study. J. Affect. Disord. 174, 522–526 (2015).
Rapaport, M. H., Clary, C., Fayyad, R. & Endicott, J. Quality-of-life impairment in depressive and anxiety disorders. Am. J. Psychiatry 162, 1171–1178 (2005).
Harvey, S. B. et al. The long-term consequences of military deployment: a 5-year cohort study of United Kingdom reservists deployed to Iraq in 2003. Am. J. Epidemiol. 176, 1177–1184 (2012).
Bosco, M. A., Gallinati, J. L. & Clark, M. E. Conceptualizing and treating comorbid chronic pain and PTSD. Pain Res. Treatment 2013, 1–10 (2013).
Kaniasty, K. & Norris, F. H. Longitudinal linkages between perceived social support and posttraumatic stress symptoms: sequential roles of social causation and social selection. J. Traumat. Stress 21, 274–281 (2008). Examining a large sample of natural disaster victims in Mexico, the authors of this study find that initially lower social support leads to greater rates of PTSD. However, over time, PTSD erodes social support, making individuals increasingly vulnerable to downstream challenges.
Yehuda, R., Lehrner, A. & Rosenbaum, T. Y. PTSD and sexual dysfunction in men and women. J. Sex. Med. 12, 1107–1119 (2015).
Hobfoll, S. E. Traumatic stress: a theory based on rapid loss of resources. Anxiety Res. 4, 187–197 (1991). The theory presented in this paper proposes that individuals experiencing traumatic stress often experience the concordant loss of personal, social and material resources, and this process occurs rapidly.
Hobfoll, S. E. Stress, Culture, and Community: the Psychology and Philosophy of Stress (Plenum, 1998).
Hall, B. J., Bonanno, G. A., Bolton, P. A. & Bass, J. K. A longitudinal investigation of changes to social resources associated with psychological distress among Kurdish torture survivors living in Northern Iraq. J. Trauma. Stress 27, 446–453 (2014).
Goodson, J. T., Lefkowitz, C. M., Helstrom, A. W. & Gawrysiak, M. J. Outcomes of prolonged exposure therapy for veterans with posttraumatic stress disorder. J. Trauma. Stress 26, 419–425 (2013).
Giacco, D., Matanov, A. & Priebe, S. Symptoms and subjective quality of life in post-traumatic stress disorder: a longitudinal study. PLoS ONE 8, e60991 (2013).
Lamarche, L. J. & De Koninck, J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J. Clin. Psychiatry 68, 1257–1270 (2007).
Daskalakis, N. P., Cohen, H., Cai, G., Buxbaum, J. D. & Yehuda, R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc. Natl Acad. Sci. USA 111, 13529–13534 (2014). This paper is important because it uses an unbiased approach to look at convergent pathways in the blood and the brain. Furthermore, this animal model separates biological differences resulting from stress exposure and individual differences in pathological behaviour.
Neylan, T. C., Schadt, E. E. & Yehuda, R. Biomarkers for combat-related PTSD: focus on molecular networks from high-dimensional data. Eur. J. Psychotraumatol. 5, 23938 (2014). This paper explains how big data and novel computational methods from converging data sets will help to identify molecular networks in PTSD. Furthermore, this paper shows how animal and human data can be used together to advance the field.
Thakur, G. S. et al. Systems biology approach to understanding post-traumatic stress disorder. Mol. Biosyst. 11, 980–993 (2015).
De Jong, J. in Broken Spirits: The Treatment of Asylum Seekers and Refugees with PTSD (eds Wilson, J. P. & Drodzek, B. ) 159–179 (Brunner/Routledge Press, 2005).
Hobfoll, S. E. Resource caravans and resource caravan passageways: a new paradigm for trauma responding. Intervention 12, 21–32 (2014).
Jewkes, R., Fulu, E., Roselli, T. & Garcia-Moreno, C. Prevalence of and factors associated with non-partner rape perpetration: findings from the UN Multi-country Cross-sectional Study on Men and Violence in Asia and the Pacific. Lancet Glob. Health 1, e208–e218 (2013).
Alegria, M. et al. Prevalence, risk, and correlates of posttraumatic stress disorder across ethnic and racial minority groups in the United States. Med. Care 51, 1114–1123 (2013).
Moisander, P. A. & Edston, E. Torture and its sequel — a comparison between victims from six countries. Forens. Sci. Int. 137, 133–140 (2003).
Wolfe, J. & Kimerling, R. in Assessing Psychological Trauma and PTSD (eds Wilson, J. P. & Keane, T. M. ) 192–238 (Guilford, 1997).
Cortina, L. M. & Kubiak, S. P. Gender and posttraumatic stress: sexual violence as an explanation for women's increased risk. J. Abnorm. Psychol. 115, 753–759 (2006).
Tolin, D. F. & Foa, E. B. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol. Bull. 132, 959–992 (2006).
Breslau, N., Chilcoat, H. D., Kessler, R. C., Peterson, E. L. & Lucia, V. C. Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol. Med. 29, 813–821 (1999).
Breslau, N. & Anthony, J. C. Gender differences in the sensitivity to posttraumatic stress disorder: an epidemiological study of urban young adults. J. Abnormal Psychol. 116, 607–611 (2007).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (American Psychiatric Association, 1987).
Hopper, J. W., Frewen, P. A., van der Kolk, B. A. & Lanius, R. A. Neural correlates of re-experiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J. Trauma. Stress 20, 713–725 (2007).
World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research (Geneva, 1992).
NICE. Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. NICE[online], (2005).
Canadian Psychiatric Association. Clinical practice guidelines: management of anxiety disorders. Canadian J. Psychiatry 51 (Suppl. 2), 9S–91S(2006).
Bajor, L. A., Ticlea, A. N. & Osser, D. N. The Psychopharmacology Algorithm Project at the Harvard South Shore Program: an update on posttraumatic stress disorder. Harvard Rev. Psychiatry 19, 240–258 (2011).
Forbes, D. et al. A guide to guidelines for the treatment of PTSD and related conditions. J. Trauma. Stress 23, 537–552 (2010).
Foa, E. B., Keane, T. M., Friedman, M. J. & Cohen, J. A. Effective treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies (Guilford Press, 2008).
Ursano, R. J. et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am. J. Psychiatry 161, 3–31 (2004).
Benedek, D. M., Friedman, M. J., Zatzick, D. & Ursano, R. J. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Psychiatry Online[online], (2009).
Bernardy, N. C. & Friedman, M. J. 2010 VA/DOD Clinical Practice Guideline for Management of Post-Traumatic Stress: how busy clinicians can best adopt updated recommendations. J. Rehabil. Res. Dev. 49, vii–viii (2012).
Forbes, D. et al. Australian guidelines for the treatment of adults with acute stress disorder and post-traumatic stress disorder. Aust. N. Z. J. Psychiatry 41, 637–648 (2007).
Acknowledgements
R.Y. is supported by grants from the US Department of Defense (DOD W81XWH-10-2-0072 and DOD W81XWH-13-1-0071) and a grant from the Lightfighter Trust Foundation (LFT2009-02-1). A.C.M. is supported in part by National Health and Medical Research Council Program Grant number 568970. C.M.N. is supported in part by US National Institutes of Health (NIH) grant R01MH093500. S.E. Hobfoll is partly supported by a grant from the National Institute of Mental Health (RO1MH073687) and the Rush Center for Urban Health Equity (NIH-NHLBI 1P50HL105189). K.C.K. is supported by grants NIH MH078928 and MH093612. T.C.N. is supported in part by a research grant that was awarded and administered by the U.S. Army Medical Research & Materiel Command (USAMRMC; TCN: W81XWH-11-2-0189) and the Mental Illness Research and Education Clinical Center of the US Veterans Health Administration. The authors would like to thank L. M. Bierer for her careful final review of the manuscript, M. E. Bowers for assistance with the development of the graphics and review of references, and H. Bader for administrative coordination and integration of multiple versions of the document.
Author information
Authors and Affiliations
Contributions
Introduction (R.Y.); Epidemiology (K.C.K. and C.W.H.); Mechanisms/pathophysiology (T.C.N., R.Y., C.M.N. and R.A.L.); Diagnosis, screening and prevention (A.C.M.); Management (E.V.); Quality of life (S. E. Hobfoll); Outlook (S. E. Hyman and R.Y.); overview of Primer (R.Y.). All authors contributed to the review and to the editing of the final manuscript.
The views expressed in this article are those of the authors and do not represent an official position of the US Army, US Department of Defense or any of the affiliated institutions listed.
Corresponding author
Ethics declarations
Competing interests
R.Y. has a patent entitled Genes Associated with Posttraumatic Stress Disorder (2012/0039,812). A.C.M. receives research funding from the Australian Department of Defense and the Australian Department of Veterans Affairs. He is a Group Captain in the RAAF specialist reserves and is an advisor to the Department of Veterans Affairs. K.C.K. has consulted for Synchroneuron Inc. and Accellient Partners. T.C.N. has consulted for Genentech and has received study medication from Actelion for a study funded by the US Department of Defense, and from Glaxo-Smith-Kline for a study funded by the US Department of Veterans Affairs. S. E. Hyman has consulted on early stage drug discovery for Novartis and Sunovion. C.W.H., E.V., R.A.L., C.M.N. and S. E. Hobfoll declare no competing interests.
Rights and permissions
About this article
Cite this article
Yehuda, R., Hoge, C., McFarlane, A. et al. Post-traumatic stress disorder. Nat Rev Dis Primers 1, 15057 (2015). https://doi.org/10.1038/nrdp.2015.57
Published:
DOI: https://doi.org/10.1038/nrdp.2015.57
This article is cited by
-
Differential recruitment of brain circuits during fear extinction in non-stressed compared to stress resilient animals
Scientific Reports (2024)
-
Post-traumatic Stress Disorder in School-age Children: A Nationwide Prospective Birth Cohort Study
Journal of Child & Adolescent Trauma (2024)
-
Basal forebrain cholinergic signaling in the basolateral amygdala promotes strength and durability of fear memories
Neuropsychopharmacology (2023)
-
Pharmacological memory modulation to augment trauma-focused psychotherapy for PTSD: a systematic review of randomised controlled trials
Translational Psychiatry (2023)
-
Fatty acid amide hydrolase inhibition alleviates anxiety-like symptoms in a rat model used to study post-traumatic stress disorder
Psychopharmacology (2023)