Key Points
-
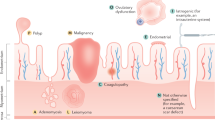
The human endometrium is a unique, dynamic tissue that is cyclically shed, repaired, regenerated and remodelled, in preparation for embryo implantation
-
Decidualization in women occurs spontaneously (regardless of the presence of an embryo) during the mid-to-late luteal phase, necessitating endometrial shedding and subsequent regeneration in the absence of conception
-
Endometrial remodelling occurs primarily under the orchestration of oestrogen and progesterone, but is influenced by many factors, including epigenetic signals and stem/progenitor cells
-
Abnormalities in endometrial remodelling lead to pathologies including infertility, endometriosis and pregnancy disorders
-
Understanding the processes that operate in the endometrium could provide information that is applicable to nonreproductive pathologies such as cancer and wound healing
Abstract
The human endometrium is a highly dynamic tissue that is cyclically shed, repaired, regenerated and remodelled, primarily under the orchestration of oestrogen and progesterone, in preparation for embryo implantation. Humans are among the very few species that menstruate and that, consequently, are equipped with unique cellular and molecular mechanisms controlling these cyclic processes. Many reproductive pathologies are specific to menstruating species, and studies in animal models rarely translate to humans. Abnormal remodelling and regeneration of the human endometrium leads to a range of reproductive complications. Furthermore, the processes regulating endometrial remodelling and implantation, including those controlling hormonal impact, breakdown and repair, stem/progenitor cell activation, inflammation and cell invasion have broad applications to other fields. This Review presents current knowledge regarding the normal and abnormal function of the human endometrium. The development of biomarkers for prediction of uterine diseases and pregnancy disorders and future avenues of investigation to improve fertility and enhance endometrial function are also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Finn, C. A. Menstruation: a nonadaptive consequence of uterine evolution. Q. Rev. Biol. 73, 163–173 (1998).
Lynch, V. J. et al. Ancient transposable elements transformed the uterine regulatory landscape and transcriptome during the evolution of mammalian pregnancy. Cell Rep. 10, 551–561 (2015).
Brosens, I. et al. The perinatal origins of major reproductive disorders in the adolescent: research avenues. Placenta 36, 341–344 (2015).
Brosens, J. J., Parker, M. G., McIndoe, A., Pijnenborg, R. & Brosens, I. A. A role for menstruation in preconditioning the uterus for successful pregnancy. Am. J. Obstet. Gynecol. 200, 615.e1–e6 (2009).
Al-Sabbagh, M., Lam, E. W. & Brosens, J. J. Mechanisms of endometrial progesterone resistance. Mol. Cell. Endocrinol. 358, 208–215 (2012).
Murphy, C. R. Uterine receptivity and the plasma membrane transformation. Cell Res. 14, 259–267 (2004).
Li, Y., Sun, X. & Dey, S. K. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep. 11, 358–365 (2015).
Revel, A. Defective endometrial receptivity. Fertil. Steril. 97, 1028–1032 (2012).
Davidson, L. M. & Coward, K. Molecular mechanisms of membrane interaction at implantation. Birth Defects Res. C Embryo Today 108, 19–32 (2016).
Dimitriadis, E. et al. Interleukin-11, IL-11 receptorα and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J. Reprod. Immunol. 69, 53–64 (2006).
Yap, J., Foo, C. F., Lee, M. Y., Stanton, P. G. & Dimitriadis, E. Proteomic analysis identifies interleukin 11 regulated plasma membrane proteins in human endometrial epithelial cells in vitro. Reprod. Biol. Endocrinol. 9, 73–87 (2011).
Haouzi, D., Dechaud, H., Assou, S., De Vos, J. & Hamamah, S. Insights into human endometrial receptivity from transcriptomic and proteomic data. Reprod. Biomed. Online 24, 23–34 (2012).
Heng, S., Hannan, N. J., Rombauts, L. J., Salamonsen, L. A. & Nie, G. PC6 levels in uterine lavage are closely associated with uterine receptivity and significantly lower in a subgroup of women with unexplained infertility. Hum. Reprod. 26, 840–846 (2011).
Heng, S. et al. Posttranslational removal of α-dystroglycan N terminus by PC5/6 cleavage is important for uterine preparation for embryo implantation in women. FASEB J. 29, 4011–4022 (2015).
Croxatto, D. et al. Stromal cells from human decidua exert a strong inhibitory effect on NK cell function and dendritic cell differentiation. PLoS ONE 9, e89006 (2014).
Murakami, K. et al. Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology 155, 4542–4553 (2014).
Teklenburg, G. et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS ONE 5, e10258 (2010).
Brosens, J. J., Hayashi, N. & White, J. O. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140, 4809–4820 (1999).
Popovici, R. M. et al. Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology 147, 5662–5675 (2006).
Garrido-Gomez, T. et al. Modeling human endometrial decidualization from the interaction between proteome and secretome. J. Clin. Endocrinol. Metab. 96, 706–716 (2011).
Estella, C. et al. miRNA signature and Dicer requirement during human endometrial stromal decidualization in vitro. PLoS ONE 7, e41080 (2012).
Gellersen, B. & Brosens, J. J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 35, 851–905 (2014).
Kuroda, K. et al. Induction of 11β-HSD 1 and activation of distinct mineralocorticoid receptor- and glucocorticoid receptor-dependent gene networks in decidualizing human endometrial stromal cells. Mol. Endocrinol. 27, 192–202 (2013).
Filant, J. & Spencer, T. E. Endometrial glands are essential for blastocyst implantation and decidualization in the mouse uterus. Biol. Reprod. 88, 93 (2013).
Filant, J. & Spencer, T. E. Uterine glands: biological roles in conceptus implantation, uterine receptivity and decidualization. Int. J. Dev. Biol. 58, 107–116 (2014).
Plaks, V. et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J. Clin. Invest. 118, 3954–3965 (2008).
Ashkar, A. A., Di Santo, J. P. & Croy, B. A. Interferon γ contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J. Exp. Med. 192, 259–270 (2000).
Gong, X. et al. Insights into the paracrine effects of uterine natural killer cells. Mol. Med. Rep. 10, 2851–2860 (2014).
Nancy, P. et al. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 336, 1317–1321 (2012).
Slayden, O. D. & Brenner, R. M. A critical period of progesterone withdrawal precedes menstruation in macaques. Reprod. Biol. Endocrinol. 4 (Suppl. 1), S6 (2006).
Garry, R., Hart, R., Karthigasu, K. A. & Burke, C. A re-appraisal of the morphological changes within the endometrium during menstruation: a hysteroscopic, histological and scanning electron microscopic study. Hum. Reprod. 24, 1393–1401 (2009).
Mote, P. A., Balleine, R. L., McGowan, E. M. & Clarke, C. L. Heterogeneity of progesterone receptors A and B expression in human endometrial glands and stroma. Hum. Reprod. 15 (Suppl. 3), 48–56 (2000).
Evans, J. & Salamonsen, L. A. Decidualized human endometrial stromal cells are sensors of hormone withdrawal in the menstrual inflammatory cascade. Biol. Reprod. 90, 14 (2014).
Sugino, N., Karube-Harada, A., Taketani, T., Sakata, A. & Nakamura, Y. Withdrawal of ovarian steroids stimulates prostaglandin F2α production through nuclear factor-κB activation via oxygen radicals in human endometrial stromal cells: potential relevance to menstruation. J. Reprod. Dev. 50, 215–225 (2004).
Evans, J. & Salamonsen, L. A. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord. 13, 277–288 (2012).
Gaide Chevronnay, H. P. et al. Spatiotemporal coupling of focal extracellular matrix degradation and reconstruction in the menstrual human endometrium. Endocrinology 150, 5094–5105 (2009).
Evans, J., Kaitu'u-Lino, T. & Salamonsen, L. A. Extracellular matrix dynamics in scar-free endometrial repair: perspectives from mouse in vivo and human in vitro studies. Biol. Reprod. 85, 511–523 (2011).
Maybin, J. A. & Critchley, H. O. Steroid regulation of menstrual bleeding and endometrial repair. Rev. Endocr. Metab. Disord. 13, 253–263 (2012).
Cousins, F. L. et al. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS ONE 9, e86378 (2014).
Evans, J. et al. Endometrial CRISP3 is regulated throughout the mouse estrous and human menstrual cycle and facilitates adhesion and proliferation of endometrial epithelial cells. Biol. Reprod. 92, 99 (2015).
Evans, J. et al. Galectin-7 is important for normal uterine repair following menstruation. Mol. Hum. Reprod. 20, 787–798 (2014).
Fan, X. et al. Dynamic regulation of Wnt7a expression in the primate endometrium: implications for postmenstrual regeneration and secretory transformation. Endocrinology 153, 1063–1069 (2012).
Gargett, C. E., Schwab, K. E. & Deane, J. A. Endometrial stem/progenitor cells: the first 10 years. Hum. Reprod. Update 22, 137–163 (2016).
Gargett, C. E., Schwab, K. E., Zillwood, R. M., Nguyen, H. P. & Wu, D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol. Reprod. 80, 1136–1145 (2009).
Schwab, K. E. & Gargett, C. E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 22, 2903–2911 (2007).
Masuda, H., Anwar, S. S., Bühring, H. J., Rao, J. R. & Gargett, C. E. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 21, 2201–2214 (2012).
Spitzer, T. L. et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol. Reprod. 86, 58 (2012).
Masuda, H. et al. Endometrial side population cells: potential adult stem/progenitor cells in endometrium. Biol. Reprod. 93, 84 (2015).
Cervelló, I. et al. Reconstruction of endometrium from human endometrial side population cell lines. PLoS ONE 6, e21221 (2011).
Miyazaki, K. et al. Stem cell-like differentiation potentials of endometrial side population cells as revealed by a newly developed in vivo endometrial stem cell assay. PLoS ONE 7, e50749 (2012).
Masuda, H. et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS ONE 5, e10387 (2010).
Ulrich, D. et al. Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng. Part A 20, 785–798 (2014).
Salamonsen, L. A., Evans, J., Nguyen, H. P. & Edgell, T. A. The microenvironment of human implantation: determinant of reproductive success. Am. J. Reprod. Immunol. 75, 218–225 (2016).
Hannan, N. J. et al. Analysis of fertility-related soluble mediators in human uterine fluid identifies VEGF as a key regulator of embryo implantation. Endocrinology 152, 4948–4956 (2011).
Robertson, S. A., Chin, P. Y., Glynn, D. J. & Thompson, J. G. Peri-conceptual cytokines—setting the trajectory for embryo implantation, pregnancy and beyond. Am. J. Reprod. Immunol. 66 (Suppl. 1), 2–10 (2011).
Vilella, F. et al. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 142, 3210–3221 (2015).
Wira, C. R., Rodriguez-Garcia, M. & Patel, M. V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 15, 217–230 (2015).
Gardner, D. K., Wale, P. L., Collins, R. & Lane, M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum. Reprod. 26, 1981–1986 (2011).
Kermack, A. J. et al. Amino acid composition of human uterine fluid: association with age, lifestyle and gynaecological pathology. Hum. Reprod. 30, 917–924 (2015).
Van Sinderen, M., Menkhorst, E., Winship, A., Cuman, C. & Dimitriadis, E. Preimplantation human blastocyst-endometrial interactions: the role of inflammatory mediators. Am. J. Reprod. Immunol. 69, 427–440 (2013).
Butler, S. A. et al. Human chorionic gonadotropin (hCG) in the secretome of cultured embryos: hyperglycosylated hCG and hCG-free beta subunit are potential markers for infertility management and treatment. Reprod. Sci. 20, 1038–1045 (2013).
Evans, J. et al. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 23, 2165–2175 (2009).
Licht, P., Fluhr, H., Neuwinger, J., Wallwiener, D. & Wildt, L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol. Cell. Endocrinol. 269, 85–92 (2007).
Paiva, P. et al. Human chorionic gonadotrophin regulates FGF2 and other cytokines produced by human endometrial epithelial cells, providing a mechanism for enhancing endometrial receptivity. Hum. Reprod. 26, 1153–1162 (2011).
Sherwin, J. R. et al. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology 148, 618–626 (2007).
Gardner, D. K. Lactate production by the mammalian blastocyst: manipulating the microenvironment for uterine implantation and invasion? Bioessays 37, 364–371 (2015).
Cuman, C. et al. Preimplantation human blastocysts release factors that differentially alter human endometrial epithelial cell adhesion and gene expression relative to IVF success. Hum. Reprod. 28, 1161–1171 (2013).
Cuman, C. et al. Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion. EBioMedicine 2, 1528–1535 (2015).
Cano, A. & Gomez, R. Mir-661: a key factor in embryo-maternal dialog with potential clinical application to predict implantation outcome? EBioMedicine 2, 1312–1313 (2015).
Sampson, J. A. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am. J. Pathol. 3, 93–110 (1927).
Han, S. J. et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 163, 960–974 (2015).
Rahmioglu, N. et al. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum. Reprod. Update 20, 702–716 (2014).
Chan, R. W., Ng, E. H. & Yeung, W. S. Identification of cells with colony-forming activity, self-renewal capacity, and multipotency in ovarian endometriosis. Am. J. Pathol. 178, 2832–2844 (2011).
Gargett, C. E. et al. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 20, 591–598 (2014).
Burney, R. O. et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148, 3814–3826 (2007).
Aghajanova, L. et al. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol. Reprod. 84, 801–815 (2011).
Salker, M. et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS ONE 5, e10287 (2010).
Savaris, R. F. et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J. Clin. Endocrinol. Metab. 96, 1737–1746 (2011).
Aghajanova, L. & Giudice, L. C. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod. Sci. 18, 229–251 (2011).
Garcia-Velasco, J. A. et al. Is endometrial receptivity transcriptomics affected in women with endometriosis? A pilot study. Reprod. Biomed. Online 31, 647–654 (2015).
Rai, P., Kota, V., Deendayal, M. & Shivaji, S. Differential proteome profiling of eutopic endometrium from women with endometriosis to understand etiology of endometriosis. J. Proteome Res. 9, 4407–4419 (2010).
Díaz, I. et al. Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study. Fertil. Steril. 74, 31–34 (2000).
Budak, E. et al. Improvements achieved in an oocyte donation program over a 10-year period: sequential increase in implantation and pregnancy rates and decrease in high-order multiple pregnancies. Fertil. Steril. 88, 342–349 (2007).
Polat, M., Boynukalin, F. K., Yarali, I., Esinler, I. & Yarali, H. Endometriosis is not associated with inferior pregnancy rates in in vitro fertilization: an analysis of 616 patients. Gynecol. Obstet. Invest. 78, 59–64 (2014).
Vernaeve, V. et al. Endometrial receptivity after oocyte donation in recipients with a history of chemotherapy and/or radiotherapy. Hum. Reprod. 22, 2863–2867 (2007).
Barnhart, K., Dunsmoor-Su, R. & Coutifaris, C. Effect of endometriosis on in vitro fertilization. Fertil. Steril. 77, 1148–1155 (2002).
Harb, H. M., Gallos, I. D., Chu, J., Harb, M. & Coomarasamy, A. The effect of endometriosis on in vitro fertilisation outcome: a systematic review and meta-analysis. BJOG 120, 1308–1320 (2013).
Hadfield, R. M., Mardon, H. J., Barlow, D. H. & Kennedy, S. H. Endometriosis in monozygotic twins. Fertil. Steril. 68, 941–942 (1997).
Dyson, M. T. et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 10, e1004158 (2014).
Fassbender, A. et al. Update on biomarkers for the detection of endometriosis. Biomed. Res. Int. 2015, 130854 (2015).
Fassbender, A. et al. World endometriosis research foundation endometriosis phenome and biobanking harmonisation project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil. Steril. 102, 1244–1253 (2014).
Kupka, M. S. et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE†. Hum. Reprod. 29, 2099–2113 (2014).
Evans, J. et al. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum. Reprod. Update 20, 808–821 (2014).
Fauser, B. C. & Devroey, P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol. Metab. 14, 236–242 (2003).
Evans, J., Hannan, N. J., Hincks, C., Rombauts, L. J. & Salamonsen, L. A. Defective soil for a fertile seed? Altered endometrial development is detrimental to pregnancy success. PLoS ONE 7, e53098 (2012).
Wilcox, A. J., Baird, D. D. & Weinberg, C. R. Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 340, 1796–1799 (1999).
Macklon, N. S., van der Gaast, M. H., Hamilton, A., Fauser, B. C. & Giudice, L. C. The impact of ovarian stimulation with recombinant FSH in combination with GnRH antagonist on the endometrial transcriptome in the window of implantation. Reprod. Sci. 15, 357–365 (2008).
Shapiro, B. S. et al. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil. Steril. 99, 389–392 (2013).
Edgell, T. A., Rombauts, L. J. & Salamonsen, L. A. Assessing receptivity in the endometrium: the need for a rapid, non-invasive test. Reprod. Biomed. Online 27, 486–496 (2013).
Vilella, F. et al. PGE2 and PGF2α concentrations in human endometrial fluid as biomarkers for embryonic implantation. J. Clin. Endocrinol. Metab. 98, 4123–4132 (2013).
Ruiz-Alonso, M. et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil. Steril. 100, 818–824 (2013).
Sirmans, S. M. & Pate, K. A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 6, 1–13 (2013).
Teede, H., Deeks, A. & Moran, L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 8, 41 (2010).
Bellver, J. et al. Endometrial gene expression in the window of implantation is altered in obese women especially in association with polycystic ovary syndrome. Fertil. Steril. 95, 2335–2341 (2011).
Li, X., Feng, Y., Lin, J. F., Billig, H. & Shao, R. Endometrial progesterone resistance and PCOS. J. Biomed. Sci. 21, 2 (2014).
Piltonen, T. T. et al. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum. Reprod. 30, 1203–1215 (2015).
Matteo, M. et al. Reduced percentage of natural killer cells associated with impaired cytokine network in the secretory endometrium of infertile women with polycystic ovary syndrome. Fertil. Steril. 94, 2222–2227 (2010).
Piltonen, T. T. et al. Mesenchymal stem/progenitors and other endometrial cell types from women with polycystic ovary syndrome (PCOS) display inflammatory and oncogenic potential. J. Clin. Endocrinol. Metab. 98, 3765–3775 (2013).
Chang, E. M. et al. Insulin resistance does not affect early embryo development but lowers implantation rate in in vitro maturation-in vitro fertilization-embryo transfer cycle. Clin. Endocrinol. (Oxf.) 79, 93–99 (2013).
Ujvari, D. et al. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum. Reprod. 29, 1526–1535 (2014).
Jakubowicz, D. J., Iuorno, M. J., Jakubowicz, S., Roberts, K. A. & Nestler, J. E. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 87, 524–529 (2002).
Palomba, S. et al. Uterine effects of metformin administration in anovulatory women with polycystic ovary syndrome. Hum. Reprod. 21, 457–465 (2006).
Mohsen, I. A., Elkattan, E., Nabil, H. & Khattab, S. Effect of metformin treatment on endometrial vascular indices in anovulatory obese/overweight women with polycystic ovarian syndrome using three-dimensional power doppler ultrasonography. J. Clin. Ultrasound 41, 275–282 (2013).
Ito-Yamaguchi, A. et al. Effects of metformin on endocrine, metabolic milieus and endometrial expression of androgen receptor in patients with polycystic ovary syndrome. Gynecol. Endocrinol. 31, 44–47 (2015).
Palomba, S. et al. Six weeks of structured exercise training and hypocaloric diet increases the probability of ovulation after clomiphene citrate in overweight and obese patients with polycystic ovary syndrome: a randomized controlled trial. Hum. Reprod. 25, 2783–2791 (2010).
Hulchiy, M., Nybacka, A., Sahlin, L. & Hirschberg, A. L. Endometrial expression of estrogen receptors and the androgen receptor in women with polycystic ovary syndrome: a lifestyle intervention study. J. Clin. Endocrinol. Metab. 101, 561–571 (2016).
Li, X. et al. Reversing the reduced level of endometrial GLUT4 expression in polycystic ovary syndrome: a mechanistic study of metformin action. Am. J. Transl. Res. 7, 574–586 (2015).
Kollmann, M. et al. Strategies to improve the outcomes of assisted reproduction in women with polycystic ovarian syndrome: a systematic review and meta-analysis. Ultrasound Obstet. Gynecol. http://dx.doi.org/10.1002/uog.15898 (2016).
Feng, L., Lin, X. F., Wan, Z. H., Hu, D. & Du, Y. K. Efficacy of metformin on pregnancy complications in women with polycystic ovary syndrome: a meta-analysis. Gynecol. Endocrinol. 31, 833–839 (2015).
Al-Biate, M. A. Effect of metformin on early pregnancy loss in women with polycystic ovary syndrome. Taiwan J. Obstet. Gynecol. 54, 266–269 (2015).
Gonzalez, D. et al. Loss of WT1 expression in the endometrium of infertile PCOS patients: a hyperandrogenic effect? J. Clin. Endocrinol. Metab. 97, 957–966 (2012).
Gargett, C. E. & Ye, L. Endometrial reconstruction from stem cells. Fertil. Steril. 98, 11–20 (2012).
Singh, N. et al. Autologous stem cell transplantation in refractory Asherman's syndrome: a novel cell based therapy. J. Hum. Reprod. Sci. 7, 93–98 (2014).
Gargett, C. E. & Healy, D. L. Generating receptive endometrium in Asherman's syndrome. J. Hum. Reprod. Sci. 4, 49–52 (2011).
Lensen, S., Sadler, L. & Farquhar, C. Endometrial scratching for subfertility: everyone's doing it. Hum. Reprod. 31, 1241–1244 (2016).
Nastri, C. O. et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database Syst. Rev. http://dx.doi.org/10.1002/14651858.CD009517.pub2 (2012).
Barash, A. et al. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil. Steril. 79, 1317–1322 (2003).
Werner, M. D. et al. Endometrial disruption does not improve implantation in patients who have failed the transfer of euploid blastocysts. J. Assist. Reprod. Genet. 32, 557–562 (2015).
Dain, L. et al. Effect of local endometrial injury on pregnancy outcomes in ovum donation cycles. Fertil. Steril. 102, 048–1054 (2014).
Yeung, T. W. et al. The effect of endometrial injury on ongoing pregnancy rate in unselected subfertile women undergoing in vitro fertilization: a randomized controlled trial. Hum. Reprod. 29, 2474–2481 (2014).
Hayashi, T. et al. Single curettage endometrial biopsy injury in the proliferative phase improves reproductive outcome of subsequent in vitro fertilization-embryo transfer cycle in infertile patients with repeated embryo implantation failure. Clin. Exp. Obstet. Gynecol. 40, 323–326 (2013).
Kitaya, K. et al. Clinical background affecting pregnancy outcome following local endometrial injury in infertile patients with repeated implantation failure. Gynecol. Endocrinol. http://dx.doi.org/10.3109/09513590.2016.1144742 (2016).
Tada, Y. et al. A pilot survey on obstetric complications in pregnant women with a history of repeated embryo implantation failure and those undergoing single local endometrial injury. Clin. Exp. Obstet. Gynecol. 42, 176–178 (2015).
Simón, C. & Bellver, J. Scratching beneath 'The Scratching Case': systematic reviews and meta-analyses, the back door for evidence-based medicine. Hum. Reprod. 29, 1618–1621 (2014).
Gibreel, A. et al. Endometrial scratching for women with previous IVF failure undergoing IVF treatment. Gynecol. Endocrinol. 31, 313–316 (2015).
Verstraelen, H. et al. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1–2 region of the 16S rRNA gene. PeerJ 4, e1602 (2016).
Mitchell, C. M. et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 212, 611.e1–611.e9 (2015).
King, A. E., Critchley, H. O. & Kelly, R. W. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol. Hum. Reprod. 6, 191–196 (2000).
Fahey, J. V. et al. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 1, 317–325 (2008).
King, A. E., Critchley, H. O. & Kelly, R. W. Innate immune defences in the human endometrium. Reprod. Biol. Endocrinol. 1, 116 (2003).
Fahey, J. V. & Wira, C. R. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J. Infect. Dis. 185, 1606–1613 (2002).
Fung, K. Y. et al. Interferon-ε protects the female reproductive tract from viral and bacterial infection. Science 339, 1088–1092 (2013).
Jirtle, R. L. & Skinner, M. K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 8, 253–262 (2007).
Munro, S. K., Farquhar, C. M., Mitchell, M. D. & Ponnampalam, A. P. Epigenetic regulation of endometrium during the menstrual cycle. Mol. Hum. Reprod. 16, 297–310 (2010).
Taft, R. J., Pang, K. C., Mercer, T. R., Dinger, M. & Mattick, J. S. Non-coding RNAs: regulators of disease. J. Pathol. 220, 126–139 (2010).
Galliano, D. & Pellicer, A. MicroRNA and implantation. Fertil. Steril. 101, 1531–1544 (2014).
Hull, M. L. & Nisenblat, V. Tissue and circulating microRNA influence reproductive function in endometrial disease. Reprod. Biomed. Online 27, 515–529 (2013).
Siristatidis, C. et al. Review: microRNAs in assisted reproduction and their potential role in IVF failure. In Vivo 29, 169–175 (2015).
Kang, Y. J. et al. MiR-145 suppresses embryo-epithelial juxtacrine communication at implantation by modulating maternal IGF1R. J. Cell Sci. 128, 804–814 (2015).
Zhang, Q. et al. MicroRNA-181a is involved in the regulation of human endometrial stromal cell decidualization by inhibiting Kruppel-like factor 12. Reprod. Biol. Endocrinol. 13, 23 (2015).
Ghazal, S. et al. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol. Med. 7, 996–1003 (2015).
Wang, H. et al. LncRNA-regulated infection and inflammation pathways associated with pregnancy loss: genome wide differential expression of lncRNAs in early spontaneous abortion. Am. J. Reprod. Immunol. 72, 359–375 (2014).
Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013).
Mathivanan, S., Ji, H. & Simpson, R. J. Exosomes: extracellular organelles important in intercellular communication. J. Proteom. 73, 1907–1920 (2010).
Simons, M. & Raposo, G. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581 (2009).
Greening, D. W., Gopal, S. K., Xu, R., Simpson, R. J. & Chen, W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 40, 72–81 (2015).
Ng, Y. H. et al. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 8, e58502 (2013).
Braundmeier, A. G., Dayger, C. A., Mehrotra, P., Belton, R. J. Jr & Nowak, R. A. EMMPRIN is secreted by human uterine epithelial cells in microvesicles and stimulates metalloproteinase production by human uterine fibroblast cells. Reprod. Sci. 19, 1292–1301 (2012).
Greening, D. W., Nguyen, H. P., Elgass, K., Simpson, R. J. & Salamonsen, L. A. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: insights into endometrial-embryo interactions. Biol. Reprod. 94, 38 (2016).
Gore, A. C. et al. Executive summary to EDC-2: The Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 36, 593–602 (2015).
Newbold, R. R. Prenatal exposure to diethylstilbestrol (DES). Fertil. Steril. 89 (Suppl. 2), e55–e56 (2008).
Taylor, H. S. Endocrine disruptors affect developmental programming of HOX gene expression. Fertil. Steril. 89 (Suppl. 2), e57–e58 (2008).
Rein, D. T., Breidenbach, M. & Curiel, D. T. Current developments in adenovirus-based cancer gene therapy. Future Oncol. 2, 137–143 (2006).
Rein, D. T. et al. Treatment of endometriosis with a VEGF-targeted conditionally replicative adenovirus. Fertil. Steril. 93, 2687–2694 (2010).
Lu, X. Y., Wu, D. C., Li, Z. J. & Chen, G. Q. Polymer nanoparticles. Prog. Mol. Biol. Transl. Sci. 104, 299–323 (2011).
Abd Ellah, N. et al. Development of non-viral, trophoblast-specific gene delivery for placental therapy. PLoS ONE 10, e0140879 (2015).
Kolonin, M. G. et al. Synchronous selection of homing peptides for multiple tissues by in vivo phage display. FASEB J. 20, 979–981 (2006).
Menkhorst, E. et al. Vaginally administered PEGylated LIF antagonist blocked embryo implantation and eliminated non-target effects on bone in mice. PLoS ONE 6, e19665 (2011).
White, C. et al. Blocking LIF action in the uterus by using a PEGylated antagonist prevents implantation: A nonhormonal contraceptive strategy. Proc. Natl Acad. Sci. USA 104, 19357–19362 (2007).
Hufnagel, D., Li, F., Cosar, E., Krikun, G. & Taylor, H. S. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin. Reprod. Med. 33, 333–340 (2015).
Hubbard, S. A. & Gargett, C. E. A cancer stem cell origin for human endometrial carcinoma? Reproduction 140, 23–32 (2010).
Chan, R. W., Kaitu'u-Lino, T. & Gargett, C. E. Role of label retaining cells in estrogen-induced endometrial regeneration. Reprod. Sci. 19, 102–114 (2012).
Chan, R. W. & Gargett, C. E. Identification of label-retaining cells in mouse endometrium. Stem Cells 24, 1529–1538 (2006).
Acknowledgements
The authors are grateful for funding from National Health and Medical Research Council (NHMRC) of Australia Project Grants to J.E., L.A.S., C.E.G., E.D. and E.M. (grants 1081944, 1085435, 1098321 and 1098332), a Senior Principal Research Fellowship to L.A.S. (grant 1002028), Senior Research Fellowships to E.D. (grant 1019826), G.N. (grant 494808) and C.E.G. (grant 1042298), a Cancer Council of Victoria Fellowship to A.W. and the Victorian Infrastructure Support Program and Australian Government NHMRC Independent Research Institute Infrastructure Support Scheme.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. J.E., L.A.S., G.N., C.E.G. and E.D. provided substantial contributions to discussions of the content. All authors contributed to writing the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Evans, J., Salamonsen, L., Winship, A. et al. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol 12, 654–667 (2016). https://doi.org/10.1038/nrendo.2016.116
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.116
This article is cited by
-
Expression profile analysis of LncRNAs and mRNAs in pre-receptive endometrium of women with polycystic ovary syndrome undergoing in vitro fertilization-embryo transfer
BMC Medical Genomics (2024)
-
Pre-eclampsia
Nature Reviews Disease Primers (2023)
-
Subtle changes in perivascular endometrial mesenchymal stem cells after local endometrial injury in recurrent implantation failure
Scientific Reports (2023)
-
MicroRNAs, small regulatory elements with significant effects on human implantation: a review
Journal of Assisted Reproduction and Genetics (2023)
-
MSX1-expression during the different phases in healthy human endometrium
Archives of Gynecology and Obstetrics (2023)