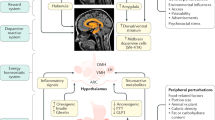
Key Points
-
Animals feed their gut bacteria, which are fully dependent on their host for providing the nutrients necessary for growth and maintenance of the bacterial population
-
Food intake is followed by cephalic reflex-mediated secretions of nutrients into the gut, which activate the gut–brain satiety pathways via release of intestinal hormones
-
Regular nutrient provision to cultured bacteria or nutrient infusion into the colon stimulates immediate bacterial growth that lasts 20 min
-
The dynamics of the regular nutrient-induced growth of bacteria are similar to the dynamics of meal-induced intestinal satiety hormone (for example, PYY) release
-
Bacterial molecules and metabolites, whose production depends on bacterial growth phases, regulate intestinal release of satiety hormones
-
Systemic bacterial molecules directly activate central appetite pathways that might integrate the energy status of both the host and its gut microbiota
Abstract
The life of all animals is dominated by alternating feelings of hunger and satiety — the main involuntary motivations for feeding-related behaviour. Gut bacteria depend fully on their host for providing the nutrients necessary for their growth. The intrinsic ability of bacteria to regulate their growth and to maintain their population within the gut suggests that gut bacteria can interfere with molecular pathways controlling energy balance in the host. The current model of appetite control is based mainly on gut–brain signalling and the animal's own needs to maintain energy homeostasis; an alternative model might also involve bacteria–host communications. Several bacterial components and metabolites have been shown to stimulate intestinal satiety pathways; at the same time, their production depends on bacterial growth cycles. This short-term bacterial growth-linked modulation of intestinal satiety can be coupled with long-term regulation of appetite, controlled by the neuropeptidergic circuitry in the hypothalamus. Indeed, several bacterial products are detected in the systemic circulation, which might act directly on hypothalamic neurons. This Review analyses the data relevant to possible involvement of the gut bacteria in the regulation of host appetite and proposes an integrative homeostatic model of appetite control that includes energy needs of both the host and its gut bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
18 November 2016
In Figure 4 of the above article published online 12 September 2016, hunger signalling to the host was incorrectly labelled as decreased, when it should have been labelled as increased. This has been corrected in the online versions of the article. We apologize for this error.
References
Turnbaugh, P. J. et al. The human microbiome project. Nature 449, 804–810 (2007).
Sekirov, I., Russell, S. L., Antunes, L. C. & Finlay, B. B. Gut microbiota in health and disease. Physiol. Rev. 90, 859–904 (2010).
Dethlefsen, L., McFall-Ngai, M. & Relman, D. A. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 449, 811–818 (2007).
Gilbert, S. F., Sapp, J. & Tauber, A. I. A symbiotic view of life: we have never been individuals. Q. Rev. Biol. 87, 325–341 (2012).
Bukharin, O. V. & Perunova, N. B. Microsymbiocenosis (UrDepart RAS, 2014).
Bäckhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host–bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Fergus, C., Barnes, D., Alqasem, M. & Kelly, V. The queuine micronutrient: charting a course from microbe to man. Nutrients 7, 2897–2929 (2015).
Sonnenburg, E. D. et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215 (2016).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Cryan, J. F. & Dinan, T. G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 (2012).
Gautron, L., Elmquist, J. K. & Williams, K. W. Neural control of energy balance: translating circuits to therapies. Cell 161, 133–145 (2015).
Aigner, M. Treasure, J., Kaye, W., Kasper, S. & The WFSBP Task Force on Eating Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of eating disorders. World J. Biol. Psychiatry 12, 400–443 (2011).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1131 (2006).
Armougom, F., Henry, M., Vialettes, B., Raccah, D. & Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in lactobacillus in obese patients and methanogens in anorexic patients. PLoS ONE 4, e7125 (2009).
Rosenbaum, M., Knight, R. & Leibel, R. L. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol. Metab. 26, 493–501 (2015).
Fetissov, S. O. & Déchelotte, P. The new link between gut–brain axis and neuropsychiatric disorders. Curr. Opin. Clin. Nutr. Metab. Care 14, 477–482 (2011).
Breton, J. et al. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab. 23, 1–11 (2016).
Thornton, S. N. Thirst and hydration: physiology and consequences of dysfunction. Physiol. Behav. 100, 15–21 (2010).
Schwartz, M. W. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Hökfelt, T., Bartfai, T. & Bloom, F. Neuropeptides: opportunities for drug discovery. Lancet Neurol. 2, 463–472 (2003).
Carter, M. E., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013).
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Norsted, E., Gömüç, B. & Meister, B. Protein components of the blood–brain barrier (BBB) in the mediobasal hypothalamus. J. Chem. Neuroanat. 36, 107–121 (2008).
Langlet, F. et al. Tanycytic VEGF-A boosts blood–hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 17, 607–617 (2013).
Schwartz, G. J. Brainstem integrative function in the central nervous system control of food intake. Forum Nutr. 63, 141–151 (2010).
Waterson, M. J. & Horvath, T. L. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 22, 962–970 (2015).
Richard, D. Cognitive and autonomic determinants of energy homeostasis in obesity. Nat. Rev. Endocrinol. 11, 489–501 (2015).
Sawchenko, P. E. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J. Comp. Neurol. 402, 435–441 (1998).
Elmquist, J. K., Maratos-Flier, E., Saper, C. B. & Flier, J. S. Unraveling the central nervous system pathways underlying responses to leptin. Nat. Neurosci. 1, 445–450 (1998).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Bumaschny, V. F. et al. Obesity-programmed mice are rescued by early genetic intervention. J. Clin. Invest. 122, 4203–4212 (2012).
Loh, K., Herzog, H. & Shi, Y.-C. Regulation of energy homeostasis by the NPY system. Trends Endocrinol. Metab. 26, 125–135 (2015).
Ollmann, M. M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997).
Hahn, T. M., Breininger, J. F., Baskin, D. G. & Schwartz, M. W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1, 271–272 (1998).
Tatemoto, K. Neuropeptide Y — a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659–660 (1982).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Poggioli, R., Vergoni, A. V. & Bertolini, A. ACTH-(1–24) and α-MSH antagonize feeding behavior stimulated by κ opiate agonists. Peptides 7, 843–848 (1986).
Harris, J. I. & Lerner, A. B. Amino-acid sequence of the α-melanocyte-stimulating hormone. Nature 179, 1346–1347 (1957).
Cone, R. D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 8, 571–578 (2005).
Broberger, C., Johansen, J., Johansson, C., Schalling, M. & Hökfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl Acad. Sci. USA 95, 15043–15048 (1998).
Zhao, R., Chen, H. & Sharp, B. M. Nicotine-induced norepinephrine release in hypothalamic paraventricular nucleus and amygdala is mediated by N-methyl-d-aspartate receptors and nitric oxide in the nucleus tractus solitarius. J. Pharmacol. Exp. Ther. 320, 837–844 (2007).
Appleyard, S. M. et al. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J. Neurosci. 25, 3578–3585 (2005).
Appleyard, S. M. et al. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J. Neurosci. 27, 13292–13302 (2007).
Larsen, P. J., Tang-Christensen, M., Holst, J. J. & Ørskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77, 257–270 (1997).
Mimee, A., Kuksis, M. & Ferguson, A. V. α-MSH exerts direct postsynaptic excitatory effects on NTS neurons and enhances GABAergic signaling in the NTS. Neuroscience 262, 70–82 (2014).
Wang, D. et al. Whole-brain mapping of the direct inputs and axonal projections of pro-opiomelanocortin (POMC) and agouti-related peptide (AgRP) neurons. Front. Neuroanat. 9, 40 (2015).
Cone, R. D. The central melanocortin system and energy homeostasis. Trends Endocrinol. Metab. 10, 211–216 (1999).
Friedman, J. M. & Mantzoros, C. S. 20 years of leptin: from the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metabolism 64, 1–4 (2015).
Hillebrand, J. J., de Wied, D. & Adan, R. A. Neuropeptides, food intake and body weight regulation: a hypothalamic focus. Peptides 23, 2283–2306 (2002).
Bouret, S. G., Draper, S. J. & Simerly, R. B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110 (2004).
Simon, J. J. et al. Neural signature of food reward processing in bulimic-type eating disorders. Soc. Cogn. Affect. Neurosci. http://dx.doi.org/10.1093/scan/nsw049 (2016).
Muenzberg-Gruening, H., Qualls-Creekmore, E., Yu, S., Morrison, C. D. & Berthoud, H.-R. Hedonics act in unison with the homeostatic system to unconsciously control body weight. Front. Nutr. 3, 6 (2016).
Denis, R G. P. et al. Palatability can drive feeding independent of AgRP neurons. Cell Metab. 22, 646–657 (2015).
Avena, N. M., Rada, P. & Hoebel, B. G. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience 156, 865–871 (2008).
Jerlhag, E. et al. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict. Biol. 12, 6–16 (2007).
Meguid, M. M. et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 16, 843–857 (2000).
Legrand, R., Lucas, N., Breton, J., Déchelotte, P. & Fetissov, S. O. Dopamine release in the lateral hypothalamus is stimulated by α-MSH in both the anticipatory and consummatory phases of feeding. Psychoneuroendocrinology 56, 79–87 (2015).
Barson, J. R., Morganstern, I. & Leibowitz, S. F. Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiol. Behav. 104, 128–137 (2011).
Norgren, R., Hajnal, A. & Mungarndee, S. S. Gustatory reward and the nucleus accumbens. Physiol. Behav. 89, 531–535 (2006).
Broberger, C. Brain regulation of food intake and appetite: molecules and networks. J. Intern. Med. 258, 301–327 (2005).
Mutt, V. in Advances in Metabolic Disorders Vol. 11 (eds Luft, R. & Levine, R.) 1–545 (Academic Press, 1988).
Wren, A. M. & Bloom, S. R. Gut hormones and appetite control. Gastroenterology 132, 2116–2130 (2007).
Janssen, S. & Depoortere, I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol. Metab. 24, 92–100 (2013).
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999).
Feinle-Bisset, C. Upper gastrointestinal sensitivity to meal-related signals in adult humans — relevance to appetite regulation and gut symptoms in health, obesity and functional dyspepsia. Physiol. Behav. 162, 69–82 (2016).
Degen, L., Matzinger, D., Drewe, J. & Beglinger, C. The effect of cholecystokinin in controlling appetite and food intake in humans. Peptides 22, 1265–1269 (2001).
Batterham, R. L. et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418, 650–654 (2002).
Drucker, D. J. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat. Clin. Pract. Endocrinol. Metab. 1, 22–31 (2005).
Flanagan, D. E. et al. The influence of insulin on circulating ghrelin. Am. J. Physiol. Endocrinol. Metab. 284, E313–E316 (2003).
Müller, T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Wynne, K. & Bloom, S. R. The role of oxyntomodulin and peptide tyrosine-tyrosine (PYY) in appetite control. Nat. Clin. Pract. Endocrinol. Metab. 2, 612–620 (2006).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Date, Y. et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123, 1120–1128 (2002).
Koda, S. The role of the vagal nerve in peripheral PYY3–36-induced feeding reduction in rats. Endocrinology 146, 2369–2375 (2005).
Cui, R. J., Li, X. & Appleyard, S. M. Ghrelin inhibits visceral afferent activation of catecholamine neurons in the solitary tract nucleus. J. Neurosci. 31, 3484–3492 (2011).
Rehfeld, J. F. Cholecystokinin as satiety signal. Int. J. Obes. (Lond.) 5, 465–469 (1981).
Wang, J. D. & Levin, P. A. Metabolism, cell growth and the bacterial cell cycle. Nat. Rev. Microbiol. 7, 822–827 (2009).
Rice, K. C. & Bayles, K. W. Molecular control of bacterial death and lysis. Microbiol. Mol. Biol. Rev. 72, 85–109 (2008).
Keller, L. & Surette, M. G. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 4, 249–258 (2006).
Freter, R., Brickner, H., Fekete, J., Vickerman, M. M. & Carey, K. E. Survival and implantation of Escherichia coli in the intestinal tract. Infect. Immun. 39, 686–703 (1983).
Flint, H. J., Scott, K. P., Louis, P. & Duncan, S. H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 9, 577–589 (2012).
Allweiss, B., Dostal, J., Carey, K. E., Edwards, T. F. & Freter, R. The role of chemotaxis in the ecology of bacterial pathogens of mucosal surfaces. Nature 266, 448–450 (1977).
Stephen, A. M. & Cummings, J. H. The microbial contribution to human faecal mass. J. Med. Microbiol. 13, 45–56 (1980).
Kotzé, S. et al. Spontaneous bacterial cell lysis and biofilm formation in the colon of the Cape Dune mole-rat and the laboratory rabbit. Appl. Microbiol. Biotechnol. 90, 1773–1783 (2011).
Roager, H. M. et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat. Microbiol. 1, 16093 (2016).
Kau, A. L., Ahern, P. P., Griffin, N. W., Goodman, A. L. & Gordon, J. I. Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336 (2011).
Kau, A. L. et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci. Transl Med 7, 276ra224 (2015).
Slack, E. et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 325, 617–620 (2009).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 1237439 (2013).
Vrieze, A. et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 60, 824–831 (2014).
Tyakht, A. V. et al. Human gut microbiota community structures in urban and rural populations in Russia. Nat. Commun. 4, 2469 (2013).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Palmer, C., Bik, E. M., DiGiulio, D. B., Relman, D. A. & Brown, P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 (2007).
Bolnick, D. I. et al. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat. Commun. 5, 4500 (2014).
Pevsner-Fischer, M. et al. Role of the microbiome in non-gastrointestinal cancers. World J. Clin. Oncol. 7, 200–213 (2016).
Haque, T. R. & Barritt, A. S. 4th. Intestinal microbiota in liver disease. Best Pract. Res. Clin. Gastroenterol. 30, 133–142 (2016).
Aron-Wisnewsky, J. & Clement, K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat. Rev. Nephrol. 12, 169–181 (2016).
Knip, M. & Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 12, 154–167 (2016).
Cotillard, A. et al. Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588 (2013).
Kleiman, S. C. et al. The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom. Med. 77, 969–981 (2015).
Woting, A. & Blaut, M. The intestinal microbiota in metabolic disease. Nutrients 8, 202 (2016).
Furet, J. P. et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59, 3049–3057 (2010).
Million, M. et al. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. (Lond.) 37, 1460–1466 (2013).
Monira, S. et al. Gut microbiota of healthy and malnourished children in Bangladesh. Front. Microbiol. 2, 228 (2011).
Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA 110, 9066–9071 (2013).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Walters, W. A., Xu, Z. & Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 588, 4223–4233 (2014).
Vijay-Kumar, M. et al. Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science 328, 228–231 (2010).
Blanton, L. V. et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science http://dx.doi.org/10.1126/science.aad3311 (2016).
Liou, A. P. et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl Med. 5, 178ra141 (2013).
Korem, T. et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 349, 1101–1106 (2015).
Thaiss, Christoph, A. et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 (2014).
Zarrinpar, A., Chaix, A., Yooseph, S. & Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 20, 1006–1017 (2014).
Liang, X., Bushman, F. D. & FitzGerald, G. A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl Acad. Sci. USA 112, 10479–10484 (2015).
Leone, V. et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17, 681–689 (2015).
Thaiss, C. A., Zeevi, D., Levy, M., Segal, E. & Elinav, E. A day in the life of the meta-organism: diurnal rhythms of the intestinal microbiome and its host. Gut Microbes 6, 137142 (2015).
Mukherji, A. et al. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc. Natl Acad. Sci. USA 112, E6691–E6698 (2015).
Fetissov, S. O. & Meguid, M. M. Serotonin delivery into the ventromedial nucleus of the hypothalamus affects differently feeding pattern and body weight in obese and lean Zucker rats. Appetite 54, 346–353 (2010).
Stunkard, A. J., Grace, W. J. & Wolff, H. G. The night-eating syndrome: a pattern of food intake among certain obese patients. Am. J. Med. 19, 78–86 (1955).
Rajpal, D. K. et al. Selective spectrum antibiotic modulation of the gut microbiome in obesity and diabetes rodent models. PLoS ONE 10, e0145499 (2016).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
de Zwaan, M., Marschollek, M. & Allison, K. C. The night eating syndrome (NES) in bariatric surgery patients. Eur. Eat. Disord. Rev. 23, 426–434 (2015).
Tremaroli, V. et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 22, 228–238 (2015).
Power, M. L. & Schulkin, J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite 50, 194–206 (2008).
Hempfling, W. P. & Mainzer, S. E. Effects of varying the carbon source limiting growth on yield and maintenance characteristics of Escherichia coli in continuous culture. J. Bacteriol. 123, 1076–1087 (1975).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Yun, A. J., Lee, P. Y., Doux, J. D. & Conley, B. R. A general theory of evolution based on energy efficiency: its implications for diseases. Med. Hypoth. 66, 664–670 (2006).
Rabot, S. et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 24, 4948–4959 (2010).
Fleissner, C. K. et al. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 104, 919–929 (2010).
Woting, A. et al. Alleviation of high fat diet-induced obesity by oligofructose in gnotobiotic mice is independent of presence of Bifidobacterium longum. Mol. Nutr. Food Res. 59, 2267–2278 (2015).
Crenn, P. et al. Net digestive absorption and adaptive hyperphagia in adult short bowel patients. Gut 53, 1279–1286 (2004).
Messing, B. et al. Intestinal absorption of free oral hyperalimentation in the very short bowel syndrome. Gastroenterology 100, 1502–1508 (1991).
Monteiro-Sepulveda, M. et al. Jejunal T cell inflammation in human obesity correlates with decreased enterocyte insulin signaling. Cell Metab. 22, 113–124 (2015).
Morita, C. et al. Gut dysbiosis in patients with anorexia nervosa. PLoS ONE 10, e0145274 (2015).
Crawford, P. A. et al. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc. Natl Acad. Sci. USA 106, 11276–11281 (2009).
Labouré, H., Van Wymelbeke, V., Fantino, M. & Nicolaidis, S. Behavioral, plasma, and calorimetric changes related to food texture modification in men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1501–R1511 (2002).
Anini, Y. et al. Comparison of the postprandial release of peptide YY and proglucagon-derived peptides in the rat. Pflügers Archiv. 438, 299–306 (1999).
Pavlov, I. P. The Work of the Digestive Glands (Charles Griffin Co. Ltd, 1902).
Gerspach, A. C., Steinert, R. E., Schönenberger, L., Graber-Maier, A. & Beglinger, C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am. J. Physiol. Endocrinol. Metab. 301, E317–E325 (2011).
Furness, J. B., Rivera, L. R., Cho, H.-J., Bravo, D. M. & Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 10, 729–740 (2013).
Cox, H. M. et al. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 11, 532–542 (2010).
Perez-Burgos, A. et al. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G211–G220 (2013).
Berthoud, H. R., Kressel, M., Raybould, H. E. & Neuhuber, W. L. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat. Embryol. (Berl.) 191, 203–212 (1995).
Ghatei, M. A., Ratcliffe, B., Bloom, S. R. & Goodlad, R. A. Fermentable dietary fibre, intestinal microflora and plasma hormones in the rat. Clin. Sci. (Lond.) 93, 109–112 (1997).
Camilleri, M., Madsen, K., Spiller, R., Van Meerveld, B. G. & Verne, G. N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 24, 503–512 (2012).
Neunlist, M. et al. The digestive neuronal–glial–epithelial unit: a new actor in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 90–100 (2012).
Hamilton, M. K., Boudry, G., Lemay, D. G. & Raybould, H. E. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G840–G851 (2015).
Bruce-Keller, A. J. et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 77, 607–615 (2015).
Ukena, S. N. et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE 2, e1308 (2007).
Ewaschuk, J. B. et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G1025–G1034 (2008).
Mariadason, J. M., Catto-Smith, A. & Gibson, P. R. Modulation of distal colonic epithelial barrier function by dietary fibre in normal rats. Gut 44, 394–399 (1999).
Wall, R. et al. in Microbial Endocrinology: The Microbiota-Gut–Brain Axis in Health and Disease (eds Lyte, M. & John Cryan, F.) 221–239 (Springer New York, 2014).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Reigstad, C. S. et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403 (2015).
Lee, H. H., Molla, M. N., Cantor, C. R. & Collins, J. J. Bacterial charity work leads to population-wide resistance. Nature 467, 82–85 (2010).
Chimerel, C. et al. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 9, 1202–1208 (2014).
Hubbard, T. D. et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 5, 12689 (2015).
Fetissov, S. O. et al. Expression of hypothalamic neuropeptides after acute TCDD treatment and distribution of Ah receptor repressor. Regul. Pept. 119, 113–124 (2004).
Bogunovic, M. et al. Enteroendocrine cells express functional Toll-like receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1770–G1783 (2007).
Palazzo, M. et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J. Immunol. 178, 42964303 (2007).
Beutler, B. & Rietschel, E. T. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3, 169–176 (2003).
Mukherji, A., Kobiita, A., Ye, T. & Chambon, P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 153, 812–827 (2013).
Zhu, X., He, L. & McCluskey, L. P. Ingestion of bacterial lipopolysaccharide inhibits peripheral taste responses to sucrose in mice. Neuroscience 258, 47–61 (2014).
Hosoi, T., Okuma, Y., Matsuda, T. & Nomura, Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Autonom. Neurosci. 120, 104–107 (2005).
Bret-Dibat, J. L., Bluthe, R. M., Kent, S., Kelley, K. W. & Dantzer, R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav. Immun. 9, 242–246 (1995).
Topping, D. L. & Clifton, P. M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064 (2001).
Canfora, E. E., Jocken, J. W. & Blaak, E. E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11, 577–591 (2015).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Chambers, E. S., Morrison, D. J. & Frost, G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc. Nutr. Soc. 74, 328–336 (2015).
Chambers, E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 (2015).
Samuel, B. S. et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl Acad. Sci. USA 105, 16767–16772 (2008).
Wanders, A. J. et al. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes. Rev. 12, 724–739 (2011).
Cani, P. D. et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 90, 1236–1243 (2009).
Tennoune, N. et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl Psychiatry 4, e458 (2014).
Tennoune, N. et al. Sex-related effects of nutritional supplementation of Escherichia coli: relevance to eating disorders. Nutrition 31, 498–507 (2015).
Lee, S. et al. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell 115, 229–240 (2003).
Haange, S. B. et al. Metaproteome analysis and molecular genetics of rat intestinal microbiota reveals section and localization resolved species distribution and enzymatic functionalities. J. Proteome Res. 11, 5406–5417 (2012).
Panaro, B. L. et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab. 20, 1018–1029 (2014).
Ericson, M. D., Schnell, S. M., Freeman, K. T. & Haskell-Luevano, C. A fragment of the Escherichia coli ClpB heat-shock protein is a micromolar melanocortin 1 receptor agonist. Bioorg. Med. Chem. Lett. 25, 5306–5308 (2015).
Maaser, C. et al. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut 55, 1415–1422 (2006).
Nwokolo, C. U., Freshwater, D. A., O'Hare, P. & Randeva, H. S. Plasma ghrelin following cure of Helicobacter pylori. Gut 52, 637–640 (2003).
Lluch, J. et al. The characterization of novel tissue microbiota using an optimized 16S metagenomic sequencing pipeline. PLoS ONE 10, e0142334 (2015).
Amar, J. et al. Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 87, 1219–1223 (2008).
Harte, A. L. et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care 35, 375–382 (2012).
Huang, Q.-H., Hruby, V. J. & Tatro, J. B. Role of central melanocortins in endotoxin-induced anorexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 276, R864–R871 (1999).
Dwarkasing, J. T., Marks, D. L., Witkamp, R. F. & van Norren, K. Hypothalamic inflammation and food intake regulation during chronic illness. Peptides 77, 60–66 (2016).
Cani, P. D. et al. Endocannabinoids — at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 12, 133–143 (2016).
Di Marzo, V. et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410, 822–825 (2001).
Jo, Y. H., Chen, Y. J., Chua, S. C. Jr, Talmage, D. A. & Role, L. W. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron 48, 1055–1066 (2005).
Nicolaidis, S. Metabolic and humoral mechanisms of feeding and genesis of the ATP/ADP/AMP concept. Physiol. Behav. 104, 8–14 (2011).
Dzamko, N. L. & Steinberg, G. R. AMPK-dependent hormonal regulation of whole-body energy metabolism. Acta Physiol. 196, 115–127 (2009).
Colldén, G., Mangano, C. & Meister, B. P2X2 purinoreceptor protein in hypothalamic neurons associated with the regulation of food intake. Neuroscience 171, 62–78 (2010).
Zilberter, Y., Zilberter, T. & Bregestovski, P. Neuronal activity in vitro and the in vivo reality: the role of energy homeostasis. Trends Pharmacol. Sci. 31, 394–401 (2010).
Mächler, P. et al. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 23, 94–102 (2016).
Le Blay, G., Michel, C., Blottière, H. M. & Cherbut, C. Prolonged intake of fructo-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J. Nutr. 129, 2231–2235 (1999).
Langhans, W. Hepatic and intestinal handling of metabolites during feeding in rats. Physiol. Behav. 49, 1203–1209 (1991).
Silberbauer, C. J., Surina-Baumgartner, D. M., Arnold, M. & Langhans, W. Prandial lactate infusion inhibits spontaneous feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R646–R653 (2000).
Frost, G. et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5, 3611 (2014).
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut–brain neural circuits. Cell 156, 84–96 (2014).
Breton, J. et al. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int. J. Eat. Disord. 49, 805–808 (2016).
Alexander, K. L., Targan, S. R. & Elson, C. O. Microbiota activation and regulation of innate and adaptive immunity. Immunol. Rev. 260, 206–220 (2014).
Garidou, L. et al. The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease. Cell Metab. 22, 100–112 (2015).
Lindner, C. et al. Diversification of memory B cells drives the continuous adaptation of secretory antibodies to gut microbiota. Nat. Immunol. 16, 880–888 (2015).
Christmann, B. S. et al. Human seroreactivity to gut microbiota antigens. J. Allergy Clin. Immunol. 136, 1378–1386 (2015).
Mankarious, S. et al. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J. Lab. Clin. Med. 112, 634–640 (1988).
Fetissov, S. O. et al. Autoantibodies against appetite-regulating peptide hormones and neuropeptides: putative modulation by gut microflora. Nutrition 24, 348–359 (2008).
Fetissov, S. O. et al. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc. Natl Acad. Sci. USA 102, 14865–14870 (2005).
Karaiskos, D. et al. Psychopathological and personality features in primary Sjogren's syndrome — associations with autoantibodies to neuropeptides. Rheumatology 49, 1762–1769 (2010).
François, M. et al. Ghrelin-reactive immunoglobulins and anxiety, depression and stress-induced cortisol response in adolescents. The TRAILS study. Prog. Neuropsychopharmacol. Biol. Psychiatry 59, 1–7 (2015).
Garcia, F. D. et al. Anti-neuropeptide Y plasma immunoglobulins in relation to mood and appetite in depressive disorder. Psychoneuroendocrinology 37, 1457–1467 (2012).
Lucas, N. et al. Effects of rabbit anti-α-melanocyte-stimulating hormone (α-MSH) immunoglobulins on α-MSH signaling related to food intake control. Neuropeptides 48, 21–27 (2014).
Lucas, N. et al. Anti-α-melanocyte-stimulating hormone autoantibodies in patients with eating disorders and melanocortin 4 receptor signaling. Eur. Neuropsychopharmacol. 24, S704–S705 (2014).
Takagi, K. et al. Anti-ghrelin immunoglobulins modulate ghrelin stability and its orexigenic effect in obese mice and humans. Nat. Commun. 4, 2685 (2013).
François, M. et al. High-fat diet increases ghrelin-expressing cells in stomach, contributing to obesity. Nutrition 32, 709–715 (2016).
Hamze Sinno, M. et al. Regulation of feeding and anxiety by α-MSH reactive autoantibodies. Psychoneuroendocrinology 34, 140–149 (2009).
McFall-Ngai, M. et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236 (2013).
Maffei, M. et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1, 1155–1161 (1995).
Everard, A. et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60, 2775–2786 (2011).
Alcock, J., Maley, C. C. & Aktipis, C. A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 36, 940–949 (2014).
Thompson, J. A., Oliveira, R. A. & Xavier, K. B. Chemical conversations in the gut microbiota. Gut Microbes 7, 163–170 (2016).
Jacobi, C. A. et al. Quorum sensing in the probiotic bacterium Escherichia coli Nissle 1917 (Mutaflor) — evidence that furanosyl borate diester (AI-2) is influencing the cytokine expression in the DSS colitis mouse model. Gut Pathog. 4, 1–10 (2012).
Wynendaele, E. et al. Quorum sensing peptides selectively penetrate the blood–brain barrier. PLoS ONE 10, e0142071 (2015).
Berg, R. D. The indigenous gastrointestinal microflora. Trends Microbiol. 4, 430–435 (1996).
Chapelot, D., Aubert, R., Marmonier, C., Chabert, M. & Louis-Sylvestre, J. An endocrine and metabolic definition of the intermeal interval in humans: evidence for a role of leptin on the prandial pattern through fatty acid disposal. Am. J. Clin. Nutr. 72, 42–431 (2000).
Acknowledgements
The author is grateful to all colleagues who participated in and inspired the reviewed research and to his wife being a fervent and pertinent critic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author is a co-founder of TargEDys.
Rights and permissions
About this article
Cite this article
Fetissov, S. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol 13, 11–25 (2017). https://doi.org/10.1038/nrendo.2016.150
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.150
This article is cited by
-
Nav1.8-expressing neurons control daily oscillations of food intake, body weight and gut microbiota in mice
Communications Biology (2024)
-
The gut microbiota modulate locomotion via vagus-dependent glucagon-like peptide-1 signaling
npj Biofilms and Microbiomes (2024)
-
Dietary fiber during gestation improves lactational feed intake of sows by modulating gut microbiota
Journal of Animal Science and Biotechnology (2023)
-
Food preferences after bariatric surgery: a review update
Internal and Emergency Medicine (2023)
-
Gut microbiota and acute kidney injury: immunological crosstalk link
International Urology and Nephrology (2023)