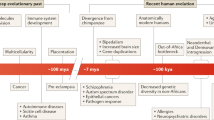
Key Points
-
We can estimate selection on traits using a measure of fitness, reliable measurements of multiple traits and the phenotypic and genetic correlations among these traits.
-
This information is available for contemporary human populations in more than 19 existing databases that cover from approximately 1,000 to more than 8 million individuals.
-
A trait under selection to decrease could respond positively, negatively or not at all, depending on the quantitative contributions of phenotypic and genetic correlations.
-
To account for traits that change with age and are influenced by the environment, traits can be expressed relative to those of other individuals of similar age and environment.
-
Phenotypic evolution can be expressed in terms of phenotypic (P) and genetic (G) variance–covariances matrices and a vector of selection gradients, β.
-
Most human traits have measurable heritability and will respond to selection if they are not constrained by phenotypic and genetic correlations with other traits.
-
Traits directly related to lifetime reproductive success tend to have lower heritabilities than traits that have a less direct relationship to fitness.
-
Selection is acting in some human populations to reduce age at first reproduction, to increase age at menopause in females and to reduce total blood cholesterol.
-
A major unresolved issue is how to deal with cultural evolution and gene–culture co-evolution.
-
One next step will be to determine whether phenotypic changes are tracked by changes in the allele frequencies of the genes associated with the traits.
Abstract
Are humans currently evolving? This question can be answered using data on lifetime reproductive success, multiple traits and genetic variation and covariation in those traits. Such data are available in existing long-term, multigeneration studies — both clinical and epidemiological — but they have not yet been widely used to address contemporary human evolution. Here we review methods to predict evolutionary change and attempts to measure selection and inheritance in humans. We also assemble examples of long-term studies in which additional measurements of evolution could be made. The evidence strongly suggests that we are evolving and that our nature is dynamic, not static.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
10 August 2010
In the version of this article initially published online, the e-mail address for Sean G. Byars was incorrect. This has now been corrected in all versions of the article.
References
Barreiro, L. B. & Quintana-Murci, L. From evolutionary genetics to human immunology: how selection shapes host defence genes. Nature Rev. Genet. 11, 17–30 (2010).
Bradley, B. J. Reconstructing phylogenies and phenotypes: a molecular view of human evolution. J. Anat. 212, 337–53 (2008).
Han, Y. et al. Evidence of positive selection on a class I ADH locus. Amer J. Hum. Genet. 80, 441–456 (2007).
Laland, K. N., Odling-Smee, J. & Myles, S. How culture shaped the human genome: bringing genetics and the human sciences together. Nature Rev. Genet. 11, 137–148 (2010).
Tishkoff, S. A. et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nature Genet. 39, 31–40 (2007). An excellent example of detecting signatures of selection in the human genome.
Perry, G. H. et al. Diet and the evolution of human amylase gene copy number variation. Nature Genet. 39, 1256–1260 (2007).
Cavalli-Sforza, L., Menozzi, P. & Piazza, A. The History and Geography of Human Genes. (Princeton University Press, Princeton, 1994).
Biswas, S. & Akey, J. Genomic insights into positive selection. Trends Genet. 22, 437–446 (2006).
Grossman, S. R. et al. A composite of multiple signals distinguishes causal variants in regions of positive selection. Science 12, 883–886 (2010).
Novembre, J. & Di Rienzo, A. Spatial patterns of variation due to natural selection in humans. Nature Rev. Genet. 10, 745–755 (2009).
Sabeti, P. et al. Positive natural selection in the human lineage. Science 312, 1614–1620 (2006).
Byars, S. G., Ewbank, D., Govindaraju, D. R. & Stearns, S. C. Natural selection in a contemporary human population. Proc. Natl Acad. Sci. USA 107, 1787–1792 (2010). By detecting significant selection on height, weight, age at first birth and menopause, this paper illustrates in detail the methods discussed in this Review.
Castelli, W. P. & Anderson, K. A population at risk. Prevalence of high cholesterol levels in hypertensive patients in the Framingham Study. Am. J. Med. 80, 23–32 (1986).
Stearns, S. C., Nesse, R. M., Govindaraju, D. R. & Ellison, P. T. Evolutionary perspectives on health and medicine. Proc. Natl Acad. Sci. USA 107, 1691–1695 (2010). A recent overview of the diverse applications of evolutionary thought to issues of medical importance.
Boomsma, D. I. Twin registers in Europe: an overview. Twin Research 1, 34–51 (1998).
Pedersen, C. B., Gotzsche, H., Moller, J. O. & Mortensen, P. B. The Danish Civil Registration System — a cohort of eight million persons. Danish Med. Bull. 53, 441–449 (2006).
Kaar, P., Jokela, J., Helle, T. & Kojola, I. Direct and correlative phenotypic selection on life-history traits in three pre-industrial human populations. Proc. R. Soc. Lond. B 263, 1475–1480 (1996).
Charlesworth, B. Evolution in Age-Structured Populations 2nd edn (Cambridge University Press, Cambridge, 1994).
McGraw, J. & Caswell, H. Estimation of individual fitness from life-history data. Am. Nat. 147, 47–64 (1996).
Schonemann, P. On models and muddles of heritability. Genetica 99, 97–108 (1997).
Stinchcombe, J. et al. Testing for environmentally induced bias in phenotypic estimates of natural selection: theory and practice. Am. Nat. 160, 511–523 (2002).
Coulson, T. & Tuljapurkar, S. The dynamics of a quantitative trait in an age-structured population living in a variable environment. Am. Nat. 172, 599–612 (2008).
Hastie, T. J. & Tibshirani, R. J. Generalized Additive Models. (Chapman and Hall, London, 1990).
Cleveland, W. S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74, 829–836 (1979).
Lande, R. & Arnold, S. The measurement of selection on correlated characters Evolution 37, 1210–1226 (1983). A classic paper in which methods were presented that transformed evolutionary quantitative genetics.
Lande, R. Quantitative genetic-analysis of multivariate evolution, applied to brain–body size allometry. Evolution 33, 402–416 (1979).
Robertson, A. A mathematical model of the culling process in dairy cattle. Anim. Prod. 8, 95–108 (1966). A prescient contribution that anticipated both Lande and Arnold's approach and Price's covariance theory and provided the basis for much of selection theory in plant and animal breeding.
Blangero, J., Almasy, L., Dyer, T. & Peterson, C. Sequential oligogenic linkage analysis routings. Solar Version 1.4.1. SOLAR [online], http://solar.sfbrgenetics.org (1999).
Abecasis, G. R., Cherny, S. S., Cookson, W. O. & Cardon, L. R. Merlin — rapid analysis of dense genetic maps using sparse gene flow trees. Nature Genet. 30, 97–101 (2002).
Neumaier, A. & Groeneveld, E. Restricted maximum likelihood estimation of covariances in sparse linear models. Genet. Selection Evolution 30, 3–26 (1998).
Pettay, J., Kruuk, L., Jokela, J. & Lummaa, V. Heritability and genetic constraints of life-history trait evolution in preindustrial humans. Proc. Natl Acad. Sci. USA 102, 2838–2843 (2005).
Blows, M. W. A tale of two matrices: multivariate approaches in evolutionary biology. J. Evol. Biol. 20, 1–8 (2007).
Brodie, E., Moore, A. & Janzen, F. Visualizing and quantifying natural selection. Trends Ecol. Evol. 10, 313–318 (1995).
Janzen, F. & Stern, H. Logistic regression for empirical studies of multivariate selection. Evolution 52, 1564–1571 (1998).
Kruuk, L. E. B. & Garant, D. A wake-up call for studies of natural selection? J. Evol. Biol. 20, 30–33 (2007).
Ovaskainen, O., Cano, J. M. & Merila, J. A Bayesian framework for comparative quantitative genetics. Proc. R. Soc. Lond. B 275, 669–678 (2008).
Schluter, D. Estimating the form of natural selection on a quantitative trait Evolution 42, 849–861 (1988).
Stinchcombe, J. R., Agrawal, A. F., Hohenlohe, P. A., Arnold, S. J. & Blows, M. W. Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing? Evolution 62, 2435–2440 (2008).
Blows, M. W. & Brooks, R. Measuring nonlinear selection. Am. Nat. 162, 815–820 (2003).
Schluter, D. & Nychka, D. Exploring fitness surfaces. Am. Nat. 143, 597–616 (1994).
Kingsolver, J. G. et al. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261 (2001). A comprehensive review of measurements of natural selection in a broad range of plants and animals.
Stearns, S. & Koella, J. The evolution of phenotypic plasticity in life history traits — predictions of reaction norms for age and size at maturity. Evolution 40, 893–913 (1986).
Sear, R., Allal, N., Mace, R. & Mcgregor, I. Height and reproductive success among Gambian women. Am. J. Hum. Biol. 16, 223–223 (2004).
Mueller, U. & Mazur, A. Evidence of unconstrained directional selection for male tallness. Behav. Ecol. Sociobiol. 50, 302–311 (2001).
Pawlowski, B., Dunbar, R. I. M. & Lipowicz, A. Evolutionary fitness — tall men have more reproductive success. Nature 403, 156–156 (2000).
Sear, R. Height and reproductive success — how a Gambian population compares with the West. Hum. Nat. 17, 405–418 (2006).
Nettle, D. Height and reproductive success in a cohort of British men. Hum. Nat. 13, 473–491 (2002).
Silventoinen, K., Lahelma, E. & Rahkonen, O. Social background, adult body-height and health. Int. J. Epidemiol. 28, 911–918 (1999).
Heliovaara, M., Makela, M., Knekt, P., Impivaara, O. & Aromaa, A. Determinants of sciatica and low-back-pain. Spine 16, 608–614 (1991).
Michaud, D. S. et al. Physical activity, obesity, height, and the risk of pancreatic cancer. J. Am. Med. Assoc. 286, 921–929 (2001).
Shors, A. R., Solomon, C., McTiernan, A. & White, E. Melanoma risk in relation to height, weight, and exercise (United States). Cancer Causes Control 12, 599–606 (2001).
Ramsden, E. A differential paradox: the controversy surrounding the Scottish mental surveys of intelligence and family size. J. Hist. Behav. Sci. 43, 109–134 (2007). A superb study in the recent history of science. This paper tells the intriguing story of how intelligence was predicted to decline under selection but in fact increased.
Visscher, P. M., Hill, W. G. & Wray, N. R. Heritability in the genomics era — concepts and misconceptions. Nature Rev. Genet. 9, 255–266 (2008). A masterly introduction to the heritability concept.
Mousseau, T. & Roff, D. Natural selection and the heritability of fitness components. Heredity 59, 181–197 (1987).
Stearns, S., De Jong, G. & Newman, B. The effects of phenotypic plasticity on genetic correlations. Trends Ecol. Evol. 6, 122–126 (1991).
Hill, W. G. Genetics. A century of corn selection. Science 307, 683–4 (2005).
Laurie, C. C. et al. The genetic architecture of response to long-term artificial selection for oil concentration in the maize kernel. Genetics 168, 2141–55 (2004). A thorough test of the additivity assumption of quantitative genetics. It demonstrates that responses to selection for oil content in corn continued for over 100 generations despite small population sizes.
Tait, L. Has the law of natural selection by survival of the fittest failed in the case of man? Dublin Quart J. Med. Sci. 47, 102–113 (1869).
Groth, P. & Weiss, B. Phenotype data: A neglected resource in biomedical research? Curr. Bioinform 1, 347–358 (2006).
Houle, D. Numbering the hairs on our heads: the shared challenge and promise of phenomics. Proc. Natl Acad. Sci. USA 107, 1793–1799 (2010).
Denny, J. C. et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene–disease associations. Bioinformatics 26, 1205–1210 (2010).
van Driel, M., Bruggeman, J., Vriend, G., Brunner, H. & Leunissen, J. A text-mining analysis of the human phenome. Eur. J. Hum. Genet. 14, 535–542 (2006).
Oti, M., Huynen, M. A. & Brunner, H. G. The biological coherence of human phenome databases. Am. J. Hum. Genet. 85, 801–808 (2009).
Bilder, R. M. Phenomics: Building scaffolds for biological hypotheses in the post-genomic era. Biol. Psychiatry 63, 439–440 (2008).
Bouchard, T. J. Genetic influence on human intelligence (Spearman's g): how much? Ann. Hum. Biol. 36, 527–544 (2009).
Austin, M. A. et al. Genetics of LDL subclass phenotypes in women twins — concordance, heritability, and commingling analysis. Arterioscler. Thromb. 13, 687–695 (1993).
Baare, W. F. C. et al. Quantitative genetic modeling of variation in human brain morphology. Cereb. Cortex 11, 816–824 (2001).
Beardsall, K. et al. Heritability of childhood weight gain from birth and risk markers for adult metabolic disease in prepubertal twins. J. Clin. Endocrinol. Metab. 94, 3708–3713 (2009).
Bella, J. N. et al. Genetic influences on aortic root size in American Indians — the Strong Heart Study. Arterioscler. Thromb. Vasc. Biol. 22, 1008–1011 (2002).
Brown, W. M. et al. Age-stratified heritability estimation in the Framingham Heart Study families. BMC Genet. 4 (Suppl. 1), 32 (2003).
Busjahn, A. et al. β-2 adrenergic receptor gene variations, blood pressure, and heart size in normal twins. Hypertension 35, 555–560 (2000).
Byard, P. J., Poosha, D. V. R. & Satyanarayana, M. Genetic and environmental determinants of height and weight in families from Andhra Pradesh, India. Hum. Biol. 57, 621–633 (1985).
Carmichael, C. M. & Mcgue, M. A cross-sectional examination of height, weight, and body-mass index in adult twins. J. Gerontol. A Biol. Sci. Med. Sci. 50, B237–B244 (1995).
Clark, P. J. The heritability of certain anthropometric characters as ascertained from measurements of twins. Am. J. Hum. Genet. 8, 49–54 (1956).
Dahlberg, G. Twin Births and Twins from a Hereditary Point of View (Tidens Tryckeri, Stockholm, 1926).
Darocha, F. J., Salzano, F. M., Callegar, S. M. & Pena, H. F. New studies on heritability of anthropometric characteristics as ascertained from twins. Acta Genet. Med. Gemellol. 21, 125–134 (1972).
de Bruin, J. P. et al. The role of genetic factors in age at natural menopause. Hum. Reprod. 16, 2014–2018 (2001).
de Oliveira, C. M., Pereira, A. C., de Andrade, M., Soler, J. M. & Krieger, J. E. Heritability of cardiovascular risk factors in a Brazilian population: Baependi Heart Study. BMC Med. Genet. 9, 1–8 (2008).
Decastro, J. M. Genetic influences on daily intake and meal patterns of humans. Physiol. Behav. 53, 777–782 (1993).
Deng, H. W. et al. A whole-genome linkage scan suggests several genomic regions potentially containing QTLs underlying the variation of stature. Am. J. Med. Genet. 113, 29–39 (2002).
Fischbein, S. Intra-pair similarity in physical growth of monozygotic and of dizygotic twins during puberty. Ann. Hum. Biol. 4, 417–430 (1977).
Furusho, T. On manifestation of genotypes responsible for stature. Hum. Biol. 40, 437–455 (1968).
Garner, C. et al. Genetic and environmental influences on left ventricular mass — a family study. Hypertension 36, 740–746 (2000).
Hammond, C. J., Snieder, H., Spector, T. D. & Gilbert, C. E. Factors affecting pupil size after dilatation: the Twin Eye Study. Br. J. Ophthalmol 84, 1173–1176 (2000).
Hansen, P. S. et al. Genetic and environmental causes of individual differences in thyroid size: a study of healthy Danish twins. J. Clin. Endocrinol. Metab. 89, 2071–2077 (2004).
Harrap, S. B., Stebbing, M., Hopper, J. L., Hoang, H. N. & Giles, G. G. Familial patterns of covariation for cardiovascular risk factors in adults — the Victorian Family Heart Study. Am. J. Epidemiol. 152, 704–715 (2000).
Hauspie, R. C., Bergman, P., Bielicki, T. & Susanne, C. Genetic variance in the pattern of the growth curve for height — a longitudinal analysis of male twins. Annals Hum. Biol. 21, 347–362 (1994).
Hawk, L. J. & Brook, C. G. D. Family resemblances of height, weight, and body fatness. Arch. Dis. Child. 54, 877–879 (1979).
Hewitt, J. K., Stunkard, A. J., Carroll, D., Sims, J. & Turner, J. R. A twin study approach towards understanding genetic contributions to body size and metabolic-rate. Acta Genet. Med. Gemellol. 40, 133–146 (1991).
Hunter, D. J., Snieder, H., March, L. & Sambrook, P. N. Genetic contribution to cartilage volume in women: a classical twin study. Rheumatology 42, 1495–1500 (2003).
Kohler, H. P. & Christensen, K. in Genetic Influences on Human Fertility and Sexuality (eds Rodgers, J. L., Rower, D. C. & Miller, W. B.) 67–84 (Kluwer, Boston, 2000).
Kohler, H. P., Rodgers, J. L. & Christensen, K. Between nurture and nature: the shifting determinants of female fertility in Danish twin cohorts. Soc. Biol. 49, 218–248 (2002).
Kosova, G., Abney, M. & Ober, C. Heritability of reproductive fitness traits in a human population. Proc. Natl Acad. Sci. USA 107, 1772–1778 (2010).
Liu, X. Q., Hanley, A. J. G. & Paterson, A. D. Genetic analysis of common factors underlying cardiovascular disease-related traits. BMC Genet. 4, S56 (2003).
Peccei, J. S. Genetic correlation between the ages of menarche and menopause. Hum. Nature 11, 43–63 (2000).
Pilia, G. et al. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2, e132 (2006).
Wang, Z. Q., Ouyang, Z., Wang, D. M. & Tang, X. L. Heritability of blood-pressure in 7-year-old to 12-year-old Chinese twins, with special reference to body size effects. Genet. Epidemiol. 7, 447–452 (1990).
Jokela, M. Physical attractiveness and reproductive success in humans: evidence from the late 20th century United States. Evol. Hum. Behav. 30, 342–350 (2009).
Wollmering, E. Wisconsin Longitudinal Study Handbook (12.10.07) (University of Wisconsin—Madison, Madison, 2007).
Nettle, D. Women's height, reproductive success and the evolution of sexual dimorphism in modern humans. Proc. R. Soc. Lond. B 269, 1919–1923 (2002).
Barker, D. J. P., Osmond, C., Forsén, T. J., Kajantie, E. & Eriksson, J. G. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 353, 1802–1809 (2005).
Pesonen, A.-K. et al. Reproductive traits following a parent–child separation trauma during childhood: a natural experiment during World War II. Am. J. Hum. Biol. 20, 345–351 (2008).
Abney, M., McPeek, M. S. & Ober, C. Estimation of variance components of quantitative traits in inbred populations. Am. J. Hum. Genet. 66, 629–650 (2000).
Glasson, E. J. et al. Perinatal factors and the development of autism — a population study. Arch. Gen. Psychiatry 61, 618–627 (2004).
Mitchell, B. D. et al. Genetic and environmental contributions to cardiovascular risk factors in Mexican Americans. The San Antonio Family Heart Study. Circulation 94, 2159–2170 (1996).
Niazi, T. N., Cannon-Albright, L. A. & Couldwell, W. T. Utah Population Database: a tool to study the hereditary element of nonsyndromic neurosurgical diseases. Neurosurg. Focus 28, E1 (2010).
Higgins, M. et al. NHLBI Family Heart Study: objectives and design. Am. J. Epidemiol. 143, 1219–1228 (1996).
Siest, G. et al. Objectives, design and recruitment of a familial and longitudinal cohort for studying gene–environment interactions in the field of cardiovascular risk: the Stanislas cohort. Clin. Chem. Lab. Med. 36, 35–42 (1998).
Helle, S., Lummaa, V. & Jokela, J. Are reproductive and somatic senescence coupled in humans? Late, but not early, reproduction correlated with longevity in historical Sami women. Proc. R. Soc. Lond. B 272, 29–37 (2005).
Helle, S. A tradeoff between reproduction and growth in contemporary Finnish women. Evol. Hum. Behav. 29, 189–195 (2008).
Kirk, K. M. et al. Natural selection and quantitative genetics of life-history traits in Western women: a twin study. Evolution 55, 423–435 (2001).
Weeden, J., Abrams, M. J., Green, M. C. & Sabini, J. Do high-status people really have fewer children? Education, income, and fertility in the contemporary US. Hum. Nat. 17, 377–392 (2006).
Kaar, P., Jokela, J., Helle, T. & Kojola, I. Direct and correlative phenotypic selection on life-history traits in three pre-industrial human populations. Proc R. Soc. Lond. B 263, 1475–1480 (1996).
Mealey, L. The relationship between social-status and biological success — a case-study of the Mormon religious hierarchy. Ethol. Sociobiol. 6, 249–257 (1985).
Bailey, S. M. & Garn, S. M. Socioeconomic interactions with physique and fertility. Hum. Biol. 51, 317–333 (1979).
Vetta, A. Fertility, physique, and intensity of selection. Hum. Biol. 47, 283–293 (1975).
Pawlowski, B., Dunbar, R. & Lipowicz, A. Evolutionary fitness — tall men have more reproductive success. Nature 403, 156–156 (2000).
Fieder, M. & Huber, S. The effects of sex and childlessness on the association between status and reproductive output in modern society. Evol. Hum. Behav. 28, 392–398 (2007).
Hopcroft, R. L. Sex, status, and reproductive success in the contemporary United States. Evol. Hum. Behav. 27, 104–120 (2006).
Fieder, M. & Huber, S. The effects of sex and childlessness on the association between status and reproductive output in modern society. Evol. Hum. Behav. 28, 392–398 (2007).
Nettle, D. & Pollet, T. V. Natural selection on male wealth in humans. Am. Nat. 172, 658–666 (2008).
Bean, F. D. & Wood, C. H. Ethnic variations in relationship between income and fertility. Demography 11, 629–640 (1974).
Mulder, M. B. On cultural and reproductive success — Kipsigis evidence. Am. Anthropol. 89, 617–634 (1987).
Low, B. S. Occupational status, landownership, and reproductive behavior in 19th-century Sweden: Tuna Parish. Am. Anthropol. 92, 457–468 (1990).
Wiessner, P. Hunting, healing, and hxaro exchange — a long-term perspective on!Kung (Ju/'hoansi) large-game hunting. Evol. Hum. Behav. 23, 407–436 (2002).
Acknowledgements
D.R.G. thanks C. Lee for hospitality and facilities. The authors thank the National Evolutionary Synthesis Center for supporting their collaboration and B.P. Stearns for detailed, constructive comments.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Longitudinal study
-
An observational study in which individuals are followed for a long period of time, often many decades, and in which the same traits are measured repeatedly.
- Pleiotropy
-
The action of a single gene on two or more distinct phenotypic characters.
- Linkage disequilibrium
-
A measure of whether alleles at two loci coexist in a population in a nonrandom fashion; one common cause is that the loci are neighbours on the same chromosome and therefore do not recombine.
- Directional selection
-
Natural selection that favours values of a quantitative trait at one extreme of the population distribution. In positive directional selection, natural selection favours values of a quantitative trait at the upper extreme of the population distribution.
- Generalized additive model
-
A statistical model that blends properties of generalized linear models with additive models (parametric or non-parametric) and is often used to estimate smoothing functions for scatter plots.
- Heritability
-
The proportion of the total phenotypic variation in a trait that can be attributed to genetic effects.
- Selection differential
-
The average superiority of the selected parents; it is expressed as the mean phenotypic value of the individuals selected as parents and expressed as a deviation from the population mean.
- Additive genetic variance
-
The part of the total genetic variation that is due to the main (or additive) effects of alleles on a phenotype. The additive variance determines the response to selection.
- Narrow-sense heritability
-
The proportion of phenotypic variation that can be accounted for by additive genetic effects. By contrast, broad-sense heritability includes effects of interactions among genes that are caused by dominance and epistasis.
- Quadratic regression
-
A quadratic regression estimates the parameters of an equation for a parabola that best fits the data. Here it is incorporated in a larger model that also has linear elements.
- Stabilizing selection
-
Natural selection that favours intermediate values of a quantitative trait.
Rights and permissions
About this article
Cite this article
Stearns, S., Byars, S., Govindaraju, D. et al. Measuring selection in contemporary human populations. Nat Rev Genet 11, 611–622 (2010). https://doi.org/10.1038/nrg2831
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2831
This article is cited by
-
Spatiotemporal variation in the vegetation cover of Peshawar Basin in response to climate change
Environmental Monitoring and Assessment (2023)
-
Culture and Evolvability: a Brief Archaeological Perspective
Journal of Archaeological Method and Theory (2023)
-
Fertility Dynamics and Life History Tactics Vary by Socioeconomic Position in a Transitioning Cohort of Postreproductive Chilean Women
Human Nature (2022)
-
Life History Is a Major Source of Adaptive Individual and Species Differences: a Critical Commentary on Zietsch and Sidari (2020)
Evolutionary Psychological Science (2021)
-
Antagonistic Pleiotropy in Human Disease
Journal of Molecular Evolution (2020)