Key Points
-
Natural killer T (NKT) cells are a developmentally and functionally distinct lineage of T cells restricted by CD1d–lipid antigen complexes. These cells branch from conventional MHC-restricted T cells at the CD4+CD8+ (double positive) stage of development in the thymus.
-
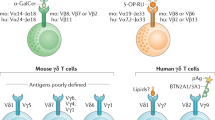
NKT-cell selection requires an interaction between a randomly generated, semi-invariant (Vα14-Jα18+) T-cell receptor (TCR) and CD1d loaded with endogenous glycolipid antigen, expressed by CD4+CD8+ thymocytes that act as selecting cells. Isoglobo-trihexosylceramide (iGb3) is a mammalian glycolipid agonist ligand for NKT cells, and some studies suggest it has a key role in NKT-cell selection. However, other studies have challenged this view and further investigation is required to resolve the issue.
-
Although the NKT-cell TCRα is invariant, a more diverse, yet still restricted range of randomly generated TCRβs can support NKT-cell selection. TCRβ contributes to the affinity of interaction with distinct glycolipid ligands and appears to endow the NKT-cell lineage with some flexibility in the types of antigen recognized.
-
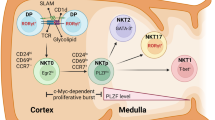
There are two crucial control points in NKT-cell development, both of which are CD1d dependent: control point 1 represents NKT-cell selection and occurs only in the thymus. Control point 2 can occur in the thymus or periphery and represents the functionally significant transition from an immature NK1.1− (CD161− in humans) phenotype to the mature NK1.1+ stage.
-
The developmental relationships between subsets of functionally distinct mature NKT cells are not well understood. Known subsets include CD4+, CD4−CD8− and CD8+ (the CD8+ NKT-cell subset is present in humans but not mice) NKT cells, but others are likely to be discovered. CD4+ NKT cells seem to be the earliest NKT cells in mice and humans, but the point at which other subsets arise, and the factors that regulate these additional differentiation steps, are not known.
-
A broad range of secreted and intracellular signalling factors have been identified that are selectively important for NKT-cell development, which supports the concept that NKT-cell differentiation is regulated independently of conventional T cells. These factors include interleukin-15 (IL-15), granulocyte/macrophage colony-stimulating factor (GM-CSF), lymphotoxin-α and lymphotoxin-β, nuclear factor-κB (NF-κB)-family members, SLAM (signalling lymphocytic activation molecule)-associated protein (SAP) and FYN.
Abstract
CD1d-dependent natural killer T (NKT) cells are a unique T-cell subset with the ability to regulate the immune system in response to a broad range of diseases. That low NKT-cell numbers are associated with many different disease states in mice and humans, combined with the fact that NKT-cell numbers vary widely between individuals, makes it crucial to understand how these cells develop and how their numbers are maintained. Here, we review the current state of knowledge of NKT-cell development and attempt to highlight the most important questions in this field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Godfrey, D. I., MacDonald, H. R., Kronenberg, M., Smyth, M. J. & Van Kaer, L. NKT cells: what's in a name? Nature Rev. Immunol. 4, 231–237 (2004). A review of the key studies that have defined the different types of NKT cell; helpful for anyone trying to understand what NKT cells are.
Godfrey, D. I. & Kronenberg, M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 (2004).
Makino, Y., Kanno, R., Koseki, H. & Taniguchi, M. Development of V-α-14+ NK T cells in the early stages of embryogenesis. Proc. Natl Acad. Sci. USA 93, 6516–6520 (1996).
Sato, H. et al. Induction of differentiation of pre-NKT cells to mature Vα14 NKT cells by granulocyte/macrophage colony-stimulating factor. Proc. Natl Acad. Sci. USA 96, 7439–7444 (1999).
Levitsky, H. I., Golumbek, P. T. & Pardoll, D. M. The fate of CD4−8− T cell receptor-αβ+ thymocytes. J. Immunol. 146, 1113–1117 (1991).
Pellicci, D. G. et al. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1− CD4+ CD1d-dependent precursor stage. J. Exp.Med. 195, 835–844 (2002).
Tilloy, F., Di Santo, J. P., Bendelac, A. & Lantz, O. Thymic dependence of invariant Vα14+ natural killer-T cell development. Eur. J. Immunol. 29, 3313–3318 (1999).
Coles, M. C. & Raulet, D. H. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 164, 2412–2418 (2000).
Hammond, K., Cain, W., Vandriel, I. & Godfrey, D. Three day neonatal thymectomy selectively depletes NK1.1+ T Cells. Int. Immunol. 10, 1491–1499 (1998).
Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. A thymic precursor to the NK T cell lineage. Science 296, 553–555 (2002).
Benlagha, K., Wei, D. G., Veiga, J., Teyton, L. & Bendelac, A. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 202, 485–492 (2005).
Gadue, P. & Stein, P. L. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J. Immunol. 169, 2397–2406 (2002). References 6, 10 and 12 provided the first clear insight into the developmental pathway that NKT cells follow as they progress from immature NK1.1− to mature NK1.1+ cells.
Gapin, L., Matsuda, J. L., Surh, C. D. & Kronenberg, M. NKT cells derive from double-positive thymocytes that are positively selected by CDId. Nature Immunol. 2, 971–978 (2001).
Egawa, T. et al. Genetic evidence supporting selection of the Vα14iNKT cell lineage from double-positive thymocyte precursors. Immunity 22, 705–716 (2005). This paper provided direct genetic evidence that NKT cells develop through the CD4+CD8+ thymocyte stage.
Bezbradica, J. S., Hill, T., Stanic, A. K., Van Kaer, L. & Joyce, S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc. Natl Acad. Sci. USA 102, 5114–5119 (2005).
Lantz, O. & Bendelac, A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180, 1097–1106 (1994).
Shimamura, M., Ohteki, T., Beutner, U. & MacDonald, H. R. Lack of directed Vα14-J-α281 rearrangements in NK1+ T cells. Eur. J. Immunol. 27, 1576–1579 (1997).
Ohteki, T. & MacDonald, H. R. Major histocompatibility complex class I related molecules control the development of CD4+8− and CD4−8− subsets of natural killer 1.1+ T cell receptor-α/β+ cells in the liver of mice. J. Exp. Med. 180, 699–704 (1994).
Coles, M. C. & Raulet, D. H. Class I dependence of the development of CD4+CD8− NK1.1+ thymocytes. J. Exp. Med. 180, 395–399 (1994).
Bendelac, A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182, 2091–2096 (1995).
Forestier, C. et al. T cell development in mice expressing CD1d directed by a classical MHC class II promoter. J. Immunol. 171, 4096–4104 (2003).
Xu, H., Chun, T., Colmone, A., Nguyen, H. & Wang, C. R. Expression of CD1d under the control of a MHC class Ia promoter skews the development of NKT cells, but not CD8+ T cells. J. Immunol. 171, 4105–4112 (2003).
Wei, D. G. et al. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J. Exp. Med. 202, 239–248 (2005). This study, together with reference 70, shows that CD1d recognition is not required for peripheral NKT-cell homing and survival, but it is required for NKT-cell maturation.
Li, W. et al. An alternate pathway for CD4 T cell development: thymocyte-expressed MHC class II selects a distinct T cell population. Immunity 23, 375–386 (2005).
Bendelac, A., Savage, P. B. & Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007). This is an up-to-date general overview of NKT-cell development and function.
Stanic, A. K. et al. Defective presentation of the CD1d1-restricted natural Vα14Jα18 NKT lymphocyte antigen caused by β-D-glucosylceramide synthase deficiency. Proc. Natl Acad. Sci. USA 100, 1849–1854 (2003).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004). This study provides several lines of evidence that the glycolipid iGb3 is a CD1d-dependent self ligand for NKT cells. It also suggests that iGb3 is the main NKT-cell selecting ligand in the thymus. This concept is controversial and has been challenged by other recent papers (references 34, 35, 37 and 38).
Schumann, J., Mycko, M. P., Dellabona, P., Casorati, G. & Macdonald, H. R. Cutting edge: influence of the TCR Vβ domain on the selection of semi-invariant NKT cells by endogenous ligands. J. Immunol. 176, 2064–2068 (2006).
Wei, D. G., Curran, S. A., Savage, P. B., Teyton, L. & Bendelac, A. Mechanisms imposing the Vβ bias of Vα14 natural killer T cells and consequences for microbial glycolipid recognition. J. Exp. Med. 203, 1197–1207 (2006).
Xia, C. et al. Synthesis and biological evaluation of α-galactosylceramide (KRN7000) and isoglobotrihexosylceramide (iGb3). Bioorg. Med. Chem. Lett. 16, 2195–2199 (2006).
Xia, C. et al. Thio-isoglobotrihexosylceramide, an agonist for activating invariant natural killer T cells. Org. Lett. 8, 5493–5496 (2006).
Cheng, L. et al. Efficient activation of Vα14 invariant NKT cells by foreign lipid antigen is associated with concurrent dendritic cell-specific self recognition. J. Immunol. 178, 2755–2762 (2007).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Gadola, S. D. et al. Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases. J. Exp. Med. 203, 2293–2303 (2006).
Schümann, J. et al. Differential alteration of lipid antigen presentation to NKT cells due to imbalances in lipid metabolism. Eur. J. Immunol. 37, 1431–1441 (2007).
Godfrey, D. I., McConville, M. J. & Pellicci, D. G. Chewing the fat on natural killer T cell development. J. Exp. Med. 203, 2229–2232 (2006).
Porubsky, S. et al. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc. Natl Acad. Sci. USA 104, 5977–5982 (2007).
Speak, A. O. et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc. Natl Acad. Sci. USA 104, 5971–5976 (2007).
Parekh, V. V. et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J. Immunol. 173, 3693–3706 (2004).
Starr, T. K., Jameson, S. C. & Hogquist, K. A. Positive and negative selection of T cells. Annu. Rev. Immunol. 21, 139–176 (2003).
Gadola, S. D., Dulphy, N., Salio, M. & Cerundolo, V. Vα24-J-αQ-independent, CD1d-restricted recognition of α-galactosylceramide by human CD4+ and CD8 αβ+ T lymphocytes. J. Immunol. 168, 5514–5520 (2002).
Matsuda, J. L. et al. Natural killer T cells reactive to a single glycolipid exhibit a highly diverse T cell receptor β repertoire and small clone size. Proc. Natl Acad. Sci. USA 98, 12636–12641 (2001).
Dellabona, P., Padovan, E., Casorati, G., Brockhaus, M. & Lanzavecchia, A. An invariant Vα24-J-αQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4−8− T cells. J. Exp. Med. 180, 1171–1176 (1994).
Porcelli, S., Yockey, C. E., Brenner, M. B. & Balk, S. P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8− α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCRα chain. J. Exp. Med. 178, 1–16 (1993).
Cantu, C. 3rd, Benlagha, K., Savage, P. B., Bendelac, A. & Teyton, L. The paradox of immune molecular recognition of α-galactosylceramide: low affinity, low specificity for CD1d, high affinity for αβ TCRs. J. Immunol. 170, 4673–4682 (2003).
Dao, T. et al. Development of CD1d-restricted NKT cells in the mouse thymus. Eur. J. Immunol. 34, 3542–3552 (2004).
Kjer-Nielsen, L. et al. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J. Exp. Med. 203, 661–673 (2006).
Schümann, J., Voyle, R. B., Wei, B. Y. & MacDonald, H. R. Cutting edge: influence of the TCR Vβ domain on the avidity of CD1d: α-galactosylceramide binding by invariant Vα14 NKT cells. J. Immunol. 170, 5815–5819 (2003).
Chun, T. et al. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J. Exp. Med. 197, 907–918 (2003).
Pellicci, D. G. et al. Intrathymic NKT cell development is blocked by the presence of α-galactosylceramide. Eur. J. Immunol. 33, 1816–1823 (2003). References 49 and 50 show that NKT cells are subject to negative selection if they develop in the presence of strong agonist ligands.
Kishimoto, H. & Sprent, J. Negative selection in the thymus includes semimature T cells. J. Exp. Med. 185, 263–271 (1997).
Stanic, A. K. et al. NF-κB controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes. J. Immunol. 172, 2265–2273 (2004).
Matsuda, J. L. et al. T-bet concomitantly controls migration, survival, and effector functions during the development of Vα14i NKT cells. Blood 107, 2797–2805 (2006).
Thedrez, A. et al. CD4 engagement by CD1d potentiates activation of CD4+ invariant NKT cells. Blood 15 March 2007 (doi:10.1182/blood-2007-01-066217).
Bendelac, A., Killeen, N., Littman, D. R. & Schwartz, R. H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science 263, 1774–1778 (1994).
Gumperz, J. E., Miyake, S., Yamamura, T. & Brenner, M. B. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195, 625–636 (2002).
Lee, P. T., Benlagha, K., Teyton, L. & Bendelac, A. Distinct functional lineages of human Va24 natural killer T cells. J. Exp. Med. 195, 637–641 (2002).
Crowe, N. Y. et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 202, 1279–1288 (2005). References 56, 57 and 58 demonstrate the existence of functionally distinct subsets of type I NKT cells in mice and humans.
Berzins, S. P., McNab, F. W., Jones, C. M., Smyth, M. J. & Godfrey, D. I. Long-term retention of mature NK1.1+ NKT cells in the thymus. J. Immunol. 176, 4059–4065 (2006).
Iwai, T. et al. Regulatory roles of NKT cells in the induction and maintenance of cyclophosphamide-induced tolerance. J. Immunol. 177, 8400–8409 (2006).
Sandberg, J. K., Stoddart, C. A., Brilot, F., Jordan, K. A. & Nixon, D. F. Development of innate CD4+ α-chain variable gene segment 24 (Vα24) natural killer T cells in the early human fetal thymus is regulated by IL-7. Proc. Natl Acad. Sci. USA 101, 7058–7063 (2004).
Baev, D. V. et al. Distinct homeostatic requirements of CD4+ and CD4− subsets of Vα24-invariant natural killer T cells in humans. Blood 104, 4150–4156 (2004).
Berzins, S. P., Cochrane, A. D., Pellicci, D. G., Smyth, M. J. & Godfrey, D. I. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur. J. Immunol. 35, 1399–1407 (2005). References 61–63 collectively show that human NKT-cell development occurs in the thymus and follows a similar maturation pathway to that of mouse NKT cells.
Davodeau, F. et al. Close phenotypic and functional similarities between human and murine αβ T cells expressing invariant TCR α-chains. J. Immunol. 158, 5603–5611 (1997).
Prussin, C. & Foster, B. TCR Vα24 and Vβ11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J. Immunol. 159, 5862–5870 (1997).
Lin, H., Nieda, M., Hutton, J. F., Rozenkov, V. & Nicol, A. J. Comparative gene expression analysis of NKT cell subpopulations. J. Leukoc. Biol. 80, 164–173 (2006).
Lin, H., Nieda, M. & Nicol, A. J. Differential proliferative response of NKT cell subpopulations to in vitro stimulation in presence of different cytokines. Eur. J. Immunol. 34, 2664–2671 (2004).
Lin, H., Nieda, M., Rozenkov, V. & Nicol, A. J. Analysis of the effect of different NKT cell subpopulations on the activation of CD4 and CD8 T cells, NK cells, and B cells. Exp. Hematol. 34, 289–295 (2006).
Zimmer, M. I. et al. A cell-type specific CD1d expression program modulates invariant NKT cell development and function. J. Immunol. 176, 1421–1430 (2006).
McNab, F. W. et al. The influence of CD1d in postselection NKT cell maturation and homeostasis. J. Immunol. 175, 3762–3768 (2005).
Matsuda, J. L. et al. Homeostasis of Vα14i NKT cells. Nature Immunol. 3, 966–974 (2002). This is the only study to directly investigate NKT-cell homeostasis in the periphery. It shows that NKT cells do not seem to occupy a specific niche, but rather, their numbers are regulated by IL-15.
Lodolce, J. P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Ohteki, T., Ho, S., Suzuki, H., Mak, T. W. & Ohashi, P. S. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J. Immunol. 159, 5931–5935 (1997).
Ranson, T. et al. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc. Natl Acad. Sci. USA 100, 2663–2668 (2003).
Ohteki, T. et al. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor- α/β+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. J. Exp. Med. 187, 967–972 (1998).
Townsend, M. J. et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 20, 477–494 (2004).
Matsuda, J. L. et al. T-bet concomitantly controls migration, survival and effector functions during the development of Vα14i NKT cells. Blood 107, 2797–2805 (2006).
Bezbradica, J. S. et al. Granulocyte-macrophage colony-stimulating factor regulates effector differentiation of invariant natural killer T cells during thymic ontogeny. Immunity 25, 487–497 (2006).
Franki, A. S. et al. A unique lymphotoxin αβ-dependent pathway regulates thymic emigration of Vα14 invariant natural killer T cells. Proc. Natl Acad. Sci. USA 103, 9160–9165 (2006).
Elewaut, D. et al. Membrane lymphotoxin is required for the development of different subpopulations of NK T cells. J. Immunol. 165, 671–679 (2000).
Iizuka, K. et al. Requirement for membrane lymphotoxin in natural killer cell development. Proc. Natl Acad. Sci. USA 96, 6336–6340 (1999).
Grech, A. P. et al. Increased thymic B cells but maintenance of thymic structure, T cell differentiation and negative selection in lymphotoxin-α and TNF gene-targeted mice. Dev. Immunol. 8, 61–74 (2000).
Elewaut, D. et al. NIK-dependent RelB activation defines a unique signaling pathway for the development of Vα14i NKT cells. J. Exp. Med. 197, 1623–1633 (2003).
Schmidt-Supprian, M. et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-κB activation. Proc. Natl Acad. Sci. USA 101, 4566–4571 (2004).
Sivakumar, V., Hammond, K. J., Howells, N., Pfeffer, K. & Weih, F. Differential requirement for Rel/nuclear factor κB family members in natural killer T cell development. J. Exp. Med. 197, 1613–1621 (2003).
Stanic, A. K. et al. Cutting edge: the ontogeny and function of Vα14Jα18 natural T lymphocytes require signal processing by protein kinase Cθ and NF-κB. J. Immunol. 172, 4667–4671 (2004).
Eberl, G., Lowin-Kropf, B. & MacDonald, H. R. Cutting edge: NKT cell development is selectively impaired in Fyn-deficient mice. J. Immunol. 163, 4091–4094 (1999).
Gadue, P., Morton, N. & Stein, P. L. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J. Exp. Med. 190, 1189–1196 (1999).
Chung, B., Aoukaty, A., Dutz, J., Terhorst, C. & Tan, R. Cutting edge: signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J. Immunol. 174, 3153–3157 (2005).
Nichols, K. E. et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nature Med. 11, 340–345 (2005).
Pasquier, B. et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J. Exp. Med. 201, 695–701(2005).
Borowski, C. & Bendelac, A. Signaling for NKT cell development: the SAP-FynT connection. J. Exp. Med. 201, 833–836 (2005).
Ma, C. S., Nichols, K. E. & Tangye, S. G. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu. Rev. Immunol. 25, 337–379 (2007).
Graham, D. B. et al. Ly9 (CD229)-deficient mice exhibit T cell defects yet do not share several phenotypic characteristics associated with SLAM- and SAP-deficient mice. J. Immunol. 176, 291–300 (2006).
Wang, N. et al. The cell surface receptor SLAM controls T cell and macrophage functions. J. Exp. Med. 199, 1255–1264 (2004).
Jordan, M. A., Fletcher, J. M., Pellicci, D. & Baxter, A. G. Slamf1, the NKT cell control gene Nkt1. J. Immunol. 178, 1618–1627 (2007).
Rocha-Campos, A. C. et al. Genetic and functional analysis of the Nkt1 locus using congenic NOD mice: improved Vα14-NKT cell performance but failure to protect against type 1 diabetes. Diabetes 55, 1163–1170 (2006).
Gadue, P., Yin, L., Jain, S. & Stein, P. L. Restoration of NK T cell development in fyn-mutant mice by a TCR reveals a requirement for Fyn during early NK T cell ontogeny. J. Immunol. 172, 6093–6100 (2004).
Joyce, S. et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science 279, 1541–1544 (1998).
Dougan, S. K., Rava, P., Hussain, M. M. & Blumberg, R. S. MTP regulated by an alternate promoter is essential for NKT cell development. J. Exp. Med. 204, 533–545 (2007).
Sagiv, Y. et al. A distal effect of microsomal triglyceride transfer protein deficiency on the lysosomal recycling of CD1d. J. Exp. Med. 204, 921–928 (2007).
Schrantz, N. et al. The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells. J. Exp. Med. 204, 841–852 (2007).
Zhou, D. et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science 303, 523–527 (2004).
Exley, M., Garcia, J., Balk, S. P. & Porcelli, S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J. Exp. Med. 186, 109–120 (1997).
Kawano, T. et al. Cd1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191, 1895–1903 (2000).
Hammond, K. J. L. et al. CD1d-restricted NKT cells: An interstrain comparison. J. Immunol. 167, 1164–1173 (2001).
Matsuda, J. L. et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192, 741–753 (2000).
Berzins, S. P. et al. Systemic NKT cell deficiency in NOD mice is not detected in peripheral blood: implications for human studies. Immunol. Cell Biol. 82, 247–252 (2004).
Lee, P. T. et al. Testing the NKT cell hypothesis of human IDDM pathogenesis. J. Clin. Invest. 110, 793–800 (2002).
Cui, J. Q. et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278, 1623–1626 (1997).
Kang, S. J. & Cresswell, P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nature Immunol. 5, 175–181 (2004).
Sagiv, Y. et al. Cutting edge: impaired glycosphingolipid trafficking and NKT cell development in mice lacking Niemann-Pick type C1 protein. J. Immunol. 177, 26–30 (2006).
Cernadas, M. et al. Lysosomal localization of murine CD1d mediated by AP-3 is necessary for NK T cell development. J. Immunol. 171, 4149–4155 (2003).
Jung, J. et al. Identification of a homozygous deletion in the AP3B1 gene causing Hermansky-Pudlak syndrome, type 2. Blood 108, 362–369 (2006).
Honey, K. et al. Thymocyte expression of cathepsin L is essential for NKT cell development. Nature Immunol. 3, 1069–1074 (2002).
Lantz, O., Sharara, L. I., Tilloy, F., Andersson, A. & Disanto, J. P. Lineage relationships and differentiation of natural killer (NK) T cells—intrathymic selection and interleukin (IL)-4 production in the absence of Nkr-P1 and Ly49 molecules. J. Exp. Med. 185, 1395–1401 (1997).
Kunisaki, Y. et al. DOCK2 Is required in T cell precursors for development of Vα14 NK T cells. J. Immunol. 176, 4640–4645 (2006).
Kim, P. J. et al. GATA-3 regulates the development and function of invariant NKT cells. J. Immunol. 177, 6650–6659 (2006).
Zullo, A. J., Benlagha, K., Bendelac, A. & Taparowsky, E. J. Sensitivity of NK1.1-negative NKT cells to transgenic BATF defines a role for activator protein-1 in the expansion and maturation of immature NKT cells in the thymus. J. Immunol. 178, 58–66 (2007).
Walunas, T. L., Wang, B., Wang, C. R. & Leiden, J. M. Cutting edge: the Ets1 transcription factor is required for the development of NK T cells in mice. J. Immunol. 164, 2857–2860 (2000).
Lacorazza, H. D. et al. The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK-T cells. Immunity 17, 437–449 (2002).
Vinay, D. S. et al. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J. Immunol. 173, 4218–4229 (2004).
Rigaud, S. et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 444, 110–114 (2006).
Schümann, J. et al. Targeted expression of human CD1d in transgenic mice reveals independent roles for thymocytes and thymic APCs in positive and negative selection of Va14i NKT cells. J. Immunol. 175, 7303–7310 (2005).
Acknowledgements
D.I.G. is the recipient of a National Health and Medical Research Council (NHMRC) Research Fellowship. S.P.B. is supported by an NHMRC Career Development Award. D.I.G. and S.P.B. are also supported by research grants from NHMRC, National Institutes of Health and the Association of International Cancer Research. We thank M. Smyth, D. Pellicci, M. Kronenberg, V. Cerundolo, S. Porubsky, A. Bendelac and H. R. MacDonald for helpful discussions during the preparation of this manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- α-GalCer–CD1d tetramers
-
Tetrameric (or multimeric) forms of CD1d molecules bound to α-galactosylceramide (α-GalCer), which have sufficient affinity for the T-cell receptor (TCR) of type I natural killer T (NKT) cells to allow the detection of invariant NKT cells by flow cytometry.
- Globo/isoglobo-series glycosphingolipids
-
One arm of the glycosphingolipid family, which is characterized by an α-linked galactose sugar in the third sugar position. Globotrihexosylceramide (Gb3) has an α1–4 linked galactose sugar, whereas isoglobotrihexosylceramide (iGb3) has an α1–3 linked galactose sugar. Additional β-linked sugars bound to these base structures yield Gb4 and iGb4.
- Agonist ligand
-
A ligand that results in cell activation and proliferation. Agonistic activity is often, but not always, associated with high-affinity and/or high-avidity binding of the T-cell receptor.
- T helper 1/ T helper 2/ T helper 0 cells
-
(TH1/TH2/TH0 cells). A classification of CD4+ T cells on the basis of the patterns of cytokines that they secrete. TH1 cells secrete interferon-γ (IFNγ) and associated pro-inflammatory cytokines that promote cell-mediated immunity. TH2 cells secrete interleukin-4 (IL-4) and associated cytokines that promote humoral immunity. TH0 cells have a 'hybrid' phenotype, being able to secrete both IFNγ and IL-4.
- Superantigen
-
A microbial protein that binds to MHC class II molecules, as well as a particular set of T-cell receptor Vβ chains, thereby leading to widespread TCR Vβ-dependent, but antigen-specificity-independent, T-cell activation.
- Nuclear factor-κB
-
(NF-κB). A family of transcription factors (including NF-κB1 (p50), NF-κB2 (p52), cRel, RelA and RelB) that regulate a range of cellular processes, including cell survival, proliferation, differentiation and cytokine production.
- Alymphoplasia
-
(aly). A mouse phenotype that is characterized by the absence of lymph nodes and Peyer's patches. It is caused by a spontaneous mutation in the gene that encodes nuclear-factor-κB-inducing kinase (NIK).
- X-linked lymphoproliferative syndrome
-
(XLP). Individuals with XLP have complicated immune dysfunctions, often triggered by infection with Epstein–Barr virus. Many patients develop fatal B-cell lymphoproliferation. The gene that encodes SLAM-associated protein (SAP) is mutated in these patients.
- Non-obese diabetic mice
-
(NOD mice). A strain of mice that normally develops idiopathic autoimmune diabetes that very closely resembles type 1 diabetes in humans. These mice have a developmental and functional deficiency in the NKT-cell compartment.
Rights and permissions
About this article
Cite this article
Godfrey, D., Berzins, S. Control points in NKT-cell development. Nat Rev Immunol 7, 505–518 (2007). https://doi.org/10.1038/nri2116
Issue Date:
DOI: https://doi.org/10.1038/nri2116
This article is cited by
-
New cell sources for CAR-based immunotherapy
Biomarker Research (2023)
-
Integrative scATAC-seq and scRNA-seq analyses map thymic iNKT cell development and identify Cbfβ for its commitment
Cell Discovery (2023)
-
Invariant natural killer T cells in lung diseases
Experimental & Molecular Medicine (2023)
-
Development of off-the-shelf hematopoietic stem cell-engineered invariant natural killer T cells for COVID-19 therapeutic intervention
Stem Cell Research & Therapy (2022)
-
SRSF1 plays a critical role in invariant natural killer T cell development and function
Cellular & Molecular Immunology (2021)