Key Points
-
The BCL-2 protein family principally determines whether a cell commits to apoptosis and hence has crucial roles in development, tissue homeostasis and immunity. Consequently, alteration of this control can either promote cancer and autoimmune diseases (too little apoptosis) or augment ischaemic conditions and contribute to degenerative disorders (too much apoptosis).
-
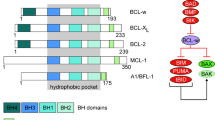
The life-or-death decision for a cell is mainly determined by the interactions between three factions of the BCL-2 family: namely, the pro-survival subfamily (for example, BCL-2, BCL-XL and MCL1) and two pro-apoptotic factions, the BH3-only proteins (for example, BIM, PUMA and BID), which convey various cytotoxic signals, and the death effectors BAX and BAK, which can convert into homo-oligomers that perforate the mitochondrial outer membrane, triggering the proteolytic cascade that demolishes the cell.
-
Biochemical and structural studies have shown that the cardinal interaction within the family is the engagement of a groove on the surface of the pro-survival family members, and of BAX and BAK, by the BH3 domain of a pro-apoptotic family member.
-
Insights have recently emerged into how BAX and BAK convert from inert globular monomers into the lethal oligomers that permeabilize the mitochondrial outer membrane. They can be activated by the binding of certain BH3 domains (particularly those of BIM and BID) to their canonical surface groove, and possibly also to a less defined rear site on BAX. The groove binding provokes BAX and BAK to undergo remarkable structural rearrangements that include release of their own BH3 domain, which can then engage the surface groove of another BAX or BAK molecule to form the 'symmetric dimers' that nucleate the larger oligomers.
-
Collectively, the manipulation of BCL-2 family genes in mice indicates that all mammalian cells are poised to commit suicide unless protected by one or more of the pro-survival family members; that nearly all cytotoxic signals, including those used in chemotherapy, are mediated through the activation of one or more BH3-only proteins; and that the activation of either BAX or BAK is necessary and sufficient for mitochondrial pathway apoptosis.
-
Novel anticancer drugs, termed 'BH3 mimetics', which engage the groove of certain pro-survival family members in a manner that is similar to that of the BH3-only proteins, have shown promise in preclinical studies and early clinical trials, particularly in patients with lymphoid malignancies, such as chronic lymphocytic leukaemia. The best characterized are navitoclax (ABT-263), which targets BCL-2, BCL-XL and BCL-W, and the new BCL-2-specific ABT-199. Their combination with other anticancer agents is likely to extend their efficacy to a wider range of malignancies.
Abstract
The BCL-2 protein family determines the commitment of cells to apoptosis, an ancient cell suicide programme that is essential for development, tissue homeostasis and immunity. Too little apoptosis can promote cancer and autoimmune diseases; too much apoptosis can augment ischaemic conditions and drive neurodegeneration. We discuss the biochemical, structural and genetic studies that have clarified how the interplay between members of the BCL-2 family on mitochondria sets the apoptotic threshold. These mechanistic insights into the functions of the BCL-2 family are illuminating the physiological control of apoptosis, the pathological consequences of its dysregulation and the promising search for novel cancer therapies that target the BCL-2 family.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Golstein, P. Cell death in us and others. Science 281, 1283 (1998).
Claveria, C., Giovinazzo, G., Sierra, R. & Torres, M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature 500, 39–44 (2013).
Vaux, D. L., Haecker, G. & Strasser, A. An evolutionary perspective on apoptosis. Cell 76, 777–779 (1994).
Kerr, J. F. R., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Brit. J. Cancer 26, 239–257 (1972).
Nagata, S., Hanayama, R. & Kawane, K. Autoimmunity and the clearance of dead cells. Cell 140, 619–630 (2010).
Hotchkiss, R. S., Strasser, A., McDunn, J. E. & Swanson, P. E. Cell death. New Engl. J. Med. 361, 1570–1583 (2009).
Yuan, J. & Kroemer, G. Alternative cell death mechanisms in development and beyond. Gene. Dev. 24, 2592–2602 (2010).
Tsujimoto, Y., Gorham, J., Cossman, J., Jaffe, E. & Croce, C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 229, 1390–1393 (1985).
Vaux, D. L., Cory, S. & Adams, J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442 (1988). The pro-survival function of BCL-2 was discovered through the finding that its overexpression prevented the death of haematopoietic cells deprived of cytokine.
McDonnell, T. J. et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 57, 79–88 (1989). This study provided the first physiological evidence that BCL-2 can control tissue homeostasis by maintaining lymphocyte survival.
Strasser, A. et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad. Sci. USA 88, 8661–8665 (1991). This study provided the first evidence that increased lymphocyte survival elicited by changes in regulation of the BCL-2 family could contribute to autoimmune disease.
Strasser, A., Harris, A. W. & Cory, S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67, 889–899 (1991).
Hengartner, M. O., Ellis, R. E. & Horvitz, H. R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 356, 494–499 (1992).
Vaux, D. L., Weissman, I. L. & Kim, S. K. Prevention of programmed cell death in Caenorhabditis elegans by human bcl-2. Science 258, 1955–1957 (1992).
Hengartner, M. O. & Horvitz, H. R. C.elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76, 665–676 (1994).
Yuan, J., Shaham, S., Ledoux, S., Ellis, H. M. & Horvitz, H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell 75, 641–652 (1993).
Zou, H., Henzel, W. J., Liu, X., Lutschg, A. & Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of Caspase-3. Cell 90, 405–413 (1997).
Yuan, J. & Horvitz, H. R. The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development 116, 309–320 (1992).
Kluck, R. M., Bossy-Wetzel, E., Green, D. R. & Newmeyer, D. D. The release of cytochrome c from mitochondria — a primary site for Bcl-2 regulation of apoptosis. Science 275, 1132–1136 (1997).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Strasser, A., Jost, P. J. & Nagata, S. The many roles of FAS receptor signaling in the immune system. Immunity 30, 180–192 (2009).
Riedl, S. J. & Salvesen, G. S. The apoptosome: signalling platform of cell death. Nature Rev. Mol. Cell Biol. 8, 405–413 (2007).
Tait, S. W. & Green, D. R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Rev. Mol. Cell Biol. 11, 621–632 (2010).
Strasser, A., Cory, S. & Adams, J. M. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 30, 3667–3683 (2011).
Letai, A. G. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nature Rev. Cancer 8, 121–132 (2008).
Martinou, J. C. & Youle, R. J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 21, 92–101 (2011).
Chipuk, J. E., Moldoveanu, T., Llambi, F., Parsons, M. J. & Green, D. R. The BCL-2 family reunion. Mol. Cell 37, 299–310 (2010).
Levine, B., Sinha, S. & Kroemer, G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4, 600–606 (2008).
Perciavalle, R. M. & Opferman, J. T. Delving deeper: MCL-1's contributions to normal and cancer biology. Trends Cell Biol. 23, 22–29 (2013).
Kvansakul, M. et al. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 15, 1564–1571 (2008).
Muchmore, S. W. et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381, 335–341 (1996).
Hinds, M. G. et al. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 14, 128–136 (2007).
Yin, X. M. et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886–891 (1999).
Kaufmann, T. et al. The BH3-only protein Bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell 129, 423–433 (2007).
Jost, P. J. et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature 460, 1035–1039 (2009).
Chou, J. J., Li, H., Salvesen, G. S., Yuan, J. & Wagner, G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96, 615–624 (1999).
McDonnell, J. M., Fushman, D., Milliman, C. L., Korsmeyer, S. J. & Cowburn, D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96, 625–634 (1999).
Adams, J. M. & Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26, 1324–1337 (2007).
Kuwana, T. et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17, 525–535 (2005).
Merino, D. et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J. Cell Biol. 186, 355–362 (2009).
Llambi, F. et al. A Unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol. Cell 44, 517–531 (2011).
Chen, L. et al. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393–403 (2005). Quantitative data revealed selectivity in the binding of BH3 domains to pro-survival BCL-2 family proteins and showed that efficient apoptosis requires the engagement of most of the expressed pro-survival proteins (see also reference 39).
Willis, S. N. et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315, 856–859 (2007). This study showed that effective induction of apoptosis requires that BH3-only proteins bind and neutralize all of the pro-survival BCL-2 family members expressed in a cell.
Willis, S. N. et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Gene. Dev. 19, 1294–1305 (2005). This study showed that activation of BAK is restrained predominantly by MCL1 and BCL-X L.
Certo, M. et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 (2006). This study introduced the concept that cells with increased levels of BCL-2 are still sensitive to BCL-2 inhibition if they have been 'primed' by stress signals that have increased the level of a bound activator BH3-only protein such as BIM.
Kim, H. et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nature Cell Biol. 8, 1348–1358 (2006).
Letai, A. et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2, 183–192 (2002). This work, using BH3 peptides, introduced the hypothesis that BH3 domains are of two classes: those that can directly activate BAX and those that can only sensitize cells to apoptosis by engaging the pro-survival family members.
Gavathiotis, E. et al. BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081 (2008).
Sattler, M. et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983–986 (1997). Building on the structure of BCL-X L (shown in reference 31), this study showed that a BAK BH3 peptide binds in the surface groove of BCL-X L , revealing a principal mode of regulation of apoptosis by this family.
Liu, X., Dai, S., Zhu, Y., Marrack, P. & Kappler, J. W. The structure of a Bcl-xL/Bim fragment complex: Implications for Bim function. Immunity 19, 341–352 (2003).
Czabotar, P. E. et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad. Sci. USA 104, 6217–6222 (2007).
Lee, E. F. et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J. Cell Biol. 180, 341–355 (2008).
Chen, T. S., Palacios, H. & Keating, A. E. Structure-based redesign of the binding specificity of anti-apoptotic Bcl-xL . J. Mol. Biol. 425, 171–185 (2013).
Lee, E. F. et al. Conformational changes in Bcl-2 pro-survival proteins determine their capacity to bind ligands. J. Biol. Chem. 284, 30508–30517 (2009).
Smits, C., Czabotar, P. E., Hinds, M. G. & Day, C. L. Structural plasticity underpins promiscuous binding of the prosurvival protein A1. Structure 16, 818–829 (2008).
Oltvai, Z. N., Milliman, C. L. & Korsmeyer, S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74, 609–619 (1993).
Wang, K., Gross, A., Waksman, G. & Korsmeyer, S. J. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell. Biol. 18, 6083–6089 (1998).
Fletcher, J. I. et al. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad. Sci. USA 105, 18081–18087 (2008).
Simmons, M. J. et al. Bfl-1/A1 functions, similar to Mcl-1, as a selective tBid and Bak antagonist. Oncogene 27, 1421–1428 (2008).
Czabotar, P. E. et al. Mutations to Bax beyond the BH3 domain disrupts interactions with pro-survival proteins and promotes apoptosis. J. Biol. Chem. 286, 7123–7131 (2011).
Ku, B., Liang, C., Jung, J. U. & Oh, B. H. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 21, 627–641 (2011).
Kim, H. et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell 36, 487–499 (2009).
Griffiths, G. J. et al. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144, 903–914 (1999).
Suzuki, M., Youle, R. J. & Tjandra, N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103, 645–654 (2000).
Edlich, F. et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 145, 104–116 (2011).
Schellenberg, B. et al. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol. Cell 49, 959–971 (2013).
Czabotar, P. E. et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531 (2013). Structural analysis showed that BAX activation requires binding of certain BH3 domains into its surface groove. It identified the residues distinguishing such BAX activators, demonstrated that BAX then unfolds into two segments and revealed that the core segment forms a BH3-in-groove symmetric dimer, which is thought to be the central element in the lethal BAX oligomers.
Gavathiotis, E., Reyna, D. E., Davis, M. L., Bird, G. H. & Walensky, L. D. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol. Cell 40, 481–492 (2010).
Leshchiner, E. S., Braun, C. R., Bird, G. H. & Walensky, L. D. Direct activation of full-length proapoptotic BAK. Proc Natl Acad. Sci. USA 110, E986–E995 (2013).
Okamoto, T. et al. Stabilizing the pro-apoptotic BimBH3 helix (BimSAHB) does not necessarily enhance affinity or biological activity. ACS Chem Biol. 8, 297–302 (2013).
Peng, R. et al. Targeting Bax interaction sites reveals that only homo-oligomerization sites are essential for its activation. Cell Death Differ. 20, 744–754 (2013).
Moldoveanu, T. et al. The x-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol. Cell 24, 677–688 (2006).
Dai, H. et al. Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J. Cell Biol. 194, 39–48 (2011).
Moldoveanu, T. et al. BID-induced structural changes in BAK promote apoptosis. Nature Struct. Mol. Biol. 20, 589–597 (2013). A BID BH3 peptide is shown to bind into the groove of BAK and elicit rearrangements similar to those in BAX (see reference 67).
Hsu, Y. T. & Youle, R. J. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272, 13829–13834 (1997).
Dewson, G. et al. To trigger apoptosis Bak exposes its BH3 domain and homo-dimerizes via BH3:grooove interactions. Mol. Cell 30, 369–380 (2008). Crosslinking studies showed that, to form oligomers, two activated BAK monomers first expose their BH3 domain and then bury it in the other monomer's surface groove, creating novel symmetric dimers, as later confirmed by structural studies on BAX (see reference 67).
Dewson, G. et al. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ. 19, 661–670 (2012).
Weber, K., Harper, N., Schwabe, J. & Cohen, G. M. BIM-Mediated Membrane Insertion of the BAK Pore Domain Is an Essential Requirement for Apoptosis. Cell Rep. 5, 409–420 (2013).
George, N. M., Evans, J. J. & Luo, X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Gene. Dev. 21, 1937–1948 (2007).
Dewson, G. et al. Bak activation for apoptosis involves oligomerization of dimers via their α6 helices. Mol. Cell 36, 696–703 (2009).
Bleicken, S. et al. Molecular details of Bax activation, oligomerization, and membrane insertion. J. Biol. Chem. 285, 6636–6647 (2010).
Zhang, Z. et al. Bax forms an oligomer via separate, yet interdependent, surfaces. J. Biol. Chem. 285, 17614–17627 (2010).
Oh, K. J. et al. Conformational changes in BAK, a pore-forming proapoptotic Bcl-2 family member, upon membrane insertion and direct evidence for the existence of BH3-BH3 contact interface in BAK homo-oligomers. J. Biol. Chem. 285, 28924–28937 (2010).
Bogner, C., Leber, B. & Andrews, D. W. Apoptosis: embedded in membranes. Curr. Opin. Cell. Biol. 22, 845–851 (2010).
Pang, Y. P. et al. Bak conformational changes induced by ligand binding: insight into BH3 domain binding and Bak homo-oligomerization. Sci. Rep. 2, 257 (2012).
Westphal, D., Dewson, G., Czabotar, P. E. & Kluck, R. M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 1813, 521–531 (2011).
Annis, M. G. et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 24, 2096–2103 (2005).
Bleicken, S., Wagner, C. & Garcia-Saez, A. J. Mechanistic differences in the membrane activity of Bax and Bcl-xL correlate with their opposing roles in apoptosis. Biophys. J. 104, 421–431 (2013).
Bleicken, S., Landeta, O., Landajuela, A., Basanez, G. & Garcia-Saez, A. J. Proapoptotic Bax and Bak form stable protein-permeable pores of tunable size. J. Biol. Chem., 288, 33241–33252 (2013)
Green, D. R. & Kroemer, G. Cytoplasmic functions of the tumour suppressor p53. Nature 458, 1127–1130 (2009).
Michalak, E. M., Villunger, A., Adams, J. M. & Strasser, A. In several cell types the tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ. 15, 1019–1029 (2008).
Chang, N. C., Nguyen, M., Germain, M. & Shore, G. C. Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1. EMBO J. 29, 606–618 (2010).
Kutuk, O. & Letai, A. Regulation of Bcl-2 family proteins by posttranslational modifications. Curr. Mol. Med. 8, 102–118 (2008).
Wei, Y., Pattingre, S., Sinha, S., Bassik, M. & Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 30, 678–688 (2008).
Dai, H. et al. Contribution of Bcl-2 phosphorylation to Bak binding and drug resistance. Cancer Res. 73, 6998–7008 (2013).
Thomas, L. W., Lam, C. & Edwards, S. W. Mcl-1; the molecular regulation of protein function. FEBS Lett. 584, 2981–2989 (2010).
Schwickart, M. et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463, 103–107 (2010).
Wertz, I. E. et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471, 110–114 (2011).
Perciavalle, R. M. et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nature Cell Biol. 14, 575–583 (2012).
Elkholi, R., Floros, K. V. & Chipuk, J. E. The role of BH3-only proteins in tumor cell development, signaling, and treatment. Genes Cancer 2, 523–537 (2011).
Herold, M. J. et al. Foxo-mediated Bim transcription is dispensable for the apoptosis of hematopoietic cells that is mediated by this BH3-only protein. EMBO Rep. 14, 992–998 (2013).
Clybouw, C. et al. Alternative splicing of Bim and Erk-mediated Bim(EL) phosphorylation are dispensable for hematopoietic homeostasis in vivo. Cell Death Differ. 19, 1060–1068 (2012).
Campbell, K. J. et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood 116, 3197–3207 (2010).
Veis, D. J., Sorenson, C. M., Shutter, J. R. & Korsmeyer, S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75, 229–240 (1993).
Bouillet, P., Cory, S., Zhang, L.-C., Strasser, A. & Adams, J. M. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev. Cell 1, 645–653 (2001).
Motoyama, N. et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science 267, 1506–1510 (1995).
Rucker, E. B. et al. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol. Endocrinol. 14, 1038–1052 (2000).
Mason, K. D. et al. Programmed anuclear cell death delimits platelet life span. Cell 128, 1173–1186 (2007).
Takehara, T. et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology 127, 1189–1197 (2004).
Akhtar, R. S., Klocke, B. J., Strasser, A. & Roth, K. A. Loss of BH3-only protein Bim inhibits apoptosis of hemopoietic cells in the fetal liver and male germ cells but not neuronal cells in bcl-x-deficient mice. J. Histochem. Cytochem. 56, 921–927 (2008).
Print, C. G. et al. Apoptosis regulator Bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad. Sci. USA 95, 12424–12431 (1998).
Ross, A. J. et al. Testicular degeneration in Bclw-deficient mice. Nature Genet. 18, 251–256 (1998).
Ross, A. J. et al. BCLW mediates survival of postmitotic Sertoli cells by regulating BAX activity. Dev. Biol. 239, 295–308 (2001).
Rinkenberger, J. L., Horning, S., Klocke, B., Roth, K. & Korsmeyer, S. J. Mcl-1 deficiency results in peri-implantation embryonic lethality. Gene. Dev. 14, 23–27 (2000).
Opferman, J. et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307, 1101–1104 (2005).
Opferman, J. T. et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426, 671–676 (2003).
Vikstrom, I. et al. Mcl-1 is essential for germinal center formation and B cell memory. Science 330, 1095–1099 (2010).
Peperzak, V. et al. Mcl-1 is essential for the survival of plasma cells. Nature Immunol. 14, 290–297 (2013).
Wang, X. et al. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Gene. Dev. 27, 1351–1364 (2013).
Thomas, R. L. et al. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Gene. Dev. 27, 1365–1377 (2013).
Hamasaki, A. et al. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J. Exp. Med. 188, 1985–1992 (1998).
Ottina, E. et al. Targeting antiapoptotic A1/Bfl-1 by in vivo RNAi reveals multiple roles in leukocyte development in mice. Blood 119, 6032–6042 (2012).
Rautureau, G. J., Day, C. L. & Hinds, M. G. The structure of Boo/Diva reveals a divergent Bcl-2 protein. Proteins 78, 2181–2186 (2010).
Youle, R. J. & Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Rev. Mol. Cell Biol. 9, 47–59 (2008).
Bouillet, P. et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 (1999). The BH3-only protein BIM was shown to be essential for specific cytotoxic responses and to maintain lymphoid homeostasis, thereby preventing autoimmunity.
Bouillet, P. et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415, 922–926 (2002).
Enders, A. et al. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreative B cells. J. Exp. Med. 198, 1119–1126 (2003).
Fischer, S. F. et al. Pro-apoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody forming cells. Blood 110, 3978–3984 (2007).
Hildeman, D. A. et al. Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity 16, 759–767 (2002).
Pellegrini, M., Belz, G., Bouillet, P. & Strasser, A. Shut down of an acute T cell immune response to viral infection is mediated by the pro-apoptotic Bcl-2 homology 3-only protein Bim. Proc. Natl Acad. Sci. USA 100, 14175–14180 (2003).
Hughes, P. D. et al. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity 28, 197–205 (2008).
Kaufmann, T. et al. Fatal hepatitis mediated by tumor necrosis factor TNFα requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity 30, 56–66 (2009).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Yu, J., Zhang, L., Hwang, P. M., Kinzler, K. W. & Vogelstein, B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7, 673–682 (2001).
Villunger, A. et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302, 1036–1038 (2003). The BH3-only proteins PUMA and to a lesser extent NOXA, which are induced by the tumour suppressor protein p53, were shown to be crucial mediators of the apoptosis elicited by genotoxic damage and certain drugs.
Jeffers, J. R. et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4, 321–328 (2003).
Shibue, T. et al. Integral role of Noxa in p53-mediated apoptotic response. Gene. Dev. 17, 2233–2238 (2003).
Naik, E., Michalak, E. M., Villunger, A., Adams, J. M. & Strasser, A. UV-radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J. Cell Biol. 176, 415–424 (2007).
Kerr, J. B. et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol. Cell 48, 343–352 (2012).
Erlacher, M. et al. BH3-only proteins Puma and Bim are rate-limiting for γ -radiation and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 106, 4131–4138 (2005).
Labi, V. et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J. Exp. Med. 205, 641–655 (2008).
Coultas, L. et al. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol. Cell. Biol. 24, 1570–1581 (2004).
Coultas, L. et al. Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis. EMBO J. 24, 3963–3973 (2005).
Ranger, A. M. et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad. Sci. USA 100, 9324–9329 (2003).
Kelly, P. N. et al. Individual and overlapping roles of BH3-only proteins Bim and Bad in apoptosis of lymphocytes and platelets and in suppression of thymic lymphoma development. Cell Death Differ. 17, 1655–1664 (2010).
Baumgartner, F. et al. Minor cell-death defects but reduced tumor latency in mice lacking the BH3-only proteins Bad and Bmf. Oncogene 32, 621–630 (2013).
Imaizumi, K. et al. Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J. Neurosci. 24, 3721–3725 (2004).
Coultas, L. et al. Hrk/DP5 contributes to the apoptosis of select neuronal populations but is dispensable for haematopoietic cell apoptosis. J. Cell Sci. 120, 2044–2052 (2007).
Erlacher, M. et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J. Exp. Med. 203, 2939–2951 (2006).
Ren, D. et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science 330, 1390–1393 (2010).
Lindsten, T. et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399 (2000). Genetic evidence that the combined absence of BAX and BAK effectively ablated intrinsic apoptosis in mice showed that they are the crucial but mutually redundant effectors of this pathway.
Mason, K. D. et al. Proapoptotic Bak and Bax guard against fatal systemic and organ-specific autoimmune disease. Proc. Natl Acad. Sci. USA 110, 2599–2604 (2013).
Zong, W. X., Lindsten, T., Ross, A. J., MacGregor, G. R. & Thompson, C. B. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Gene. Dev. 15, 1481–1486 (2001).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001). Together with the physiological results showing that apoptosis requires BAX or BAK (see reference 151), this study and reference 153 showed that BH3-only proteins must function upstream of and through BAX and BAK.
Ke, F. et al. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ. 19, 915–925 (2012).
Ke, F. et al. Consequences of the combined loss of BOK and BAK or BOK and BAX. Cell Death Dis. 4, e650 (2013).
McDonnell, T. J. & Korsmeyer, S. J. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18). Nature 349, 254–256 (1991).
Strasser, A., Harris, A. W. & Cory, S. Eμ-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells. Oncogene 8, 1–9 (1993).
Strasser, A., Harris, A. W., Bath, M. L. & Cory, S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348, 331–333 (1990). Extending the in vitro analysis in reference 9, the demonstration that BCL-2 accelerated MYC-driven tumour development provided the first direct evidence in vivo that BCL-2 overexpression is tumorigenic.
Egle, A., Harris, A. W., Bouillet, P. & Cory, S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad. Sci. USA 101, 6164–6169 (2004).
Garrison, S. P. et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol. Cell. Biol. 28, 5391–5402 (2008).
Michalak, E. M. et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ. 16, 684–696 (2009).
Frenzel, A. et al. Suppression of B-cell lymphomagenesis by the BH3-only proteins Bmf and Bad. Blood 115, 995–1005 (2010).
Eischen, C. M., Roussel, M. F., Korsmeyer, S. J. & Cleveland, J. L. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol. Cell. Biol. 21, 7653–7662 (2001).
Tsujimoto, Y., Finger, L. R., Yunis, J., Nowell, P. C. & Croce, C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226, 1097–1099 (1984).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Tagawa, H. et al. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene 24, 1348–1358 (2005).
Zantl, N. et al. Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene 26, 7038–7048 (2007).
Goodnow, C. C. Multistep pathogenesis of autoimmune disease. Cell 130, 25–35 (2007).
Rathmell, J. C., Lindsten, T., Zong, W.-X., Cinalli, R. M. & Thompson, C. B. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nature Immunol. 3, 932–939 (2002).
Happo, L. et al. Maximal killing of lymphoma cells by DNA-damage inducing therapy requires not only the p53 targets Puma and Noxa but also Bim. Blood 116, 5256–5267 (2010).
Bachmann, P. S. et al. Epigenetic silencing of BIM in glucocorticoid poor-responsive pediatric acute lymphoblastic leukemia, and its reversal by histone deacetylase inhibition. Blood 116, 3013–3022 (2010).
Tan, T. T. et al. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell 7, 227–238 (2005).
Kuroda, J. et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad. Sci. USA 103, 14907–14912 (2006).
Cragg, M. S., Kuroda, J., Puthalakath, H., Huang, D. C. S. & Strasser, A. Gefitinib-induced killing of NSCLC cell cines expressing mutant EGFR requires Bim and can be enhanced by BH3 mimetics. PLoS Med. 4, 1681–1689 (2007).
Costa, D. B. et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 4, e315 (2007).
Cragg, M. S., Jansen, E. S., Cook, M., Strasser, A. & Scott, C. L. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic J. Clin. Invest. 118, 3651–3659 (2008).
Ng, K. P. et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nature Med. 18, 521–528 (2012).
Del Gaizo Moore, V. et al. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Invest. 117, 112–121 (2007).
Merino, D. et al. Bcl-2, Bcl-xL, and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 119, 5807–5816 (2012).
Chonghaile, T. N. et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334, 1129–1133 (2011).
Vo, T. T. et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151, 344–355 (2012).
van Delft, M. F. et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10, 389–399 (2006).
Lessene, G., Czabotar, P. E. & Colman, P. M. BCL-2 family antagonists for cancer therapy. Nature Rev. Drug Discov. 7, 989–1000 (2008).
Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005). The development of ABT-737 provided the first compelling evidence that BH3 mimetic therapy for cancer was likely to become a reality.
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008). The orally bioavailable derivative of ABT-737, ABT-263 (now called navitoclax), has shown promise in early clinical trials.
Roberts, A. W. et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 30, 488–496 (2012).
Mason, K. D. et al. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad. Sci. USA 105, 17961–17966 (2008).
Tan, N. et al. Navitoclax enhances the efficacy of taxanes in non-small cell lung cancer models. Clin. Cancer Res. 17, 1394–1404 (2011).
Cragg, M. S., Harris, C., Strasser, A. & Scott, C. L. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nature Rev. Cancer 9, 321–326 (2009).
Gores, G. J. & Kaufmann, S. H. Selectively targeting Mcl-1 for the treatment of acute myelogenous leukemia and solid tumors. Gene. Dev. 26, 305–311 (2012).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature Med. 19, 202–208 (2013). Preclinical studies and early results from the first clinical trial indicate that ABT-199, the first potent and highly selective BCL-2 antagonist, has considerable promise, particularly for the therapy of lymphoid malignancies.
Vandenberg, C. J. & Cory, S. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood 121, 2285–2288 (2013).
Vaillant, F. et al. Targeting BCL-2 with the BH3 Mimetic ABT-199 in estrogen eeceptor-positive breast cancer. Cancer Cell 24, 120–129 (2013).
Lessene, G. et al. Structure-guided design of a selective BCL-XL inhibitor. Nature Chem. Biol. 9, 390–397 (2013).
Friberg, A. et al. Discovery of potent myeloid cell leukemia 1 (mcl-1) inhibitors using fragment-based methods and structure-based design. J. Med. Chem. 56, 15–30 (2013).
Placzek, W. J. et al. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 1, e40 (2010).
Glaser, S. et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Gene. Dev. 26, 120–125 (2012).
Boisvert-Adamo, K., Longmate, W., Abel, E. V. & Aplin, A. E. Mcl-1 is required for melanoma cell resistance to anoikis. Mol. Cancer Res. 7, 549–556 (2009).
Boersma, M. D. et al. Evaluation of diverse α/β-backbone patterns for functional α-helix mimicry: analogues of the Bim BH3 domain. J. Am. Chem. Soc. 134, 315–323 (2012).
LaBelle, J. L. et al. A stapled BIM BH3 helix overcomes the apoptotic resistance of refractory hematologic cancers. J. Clin. Invest. 122, 2018–2031 (2013).
Stewart, M. L., Fire, E., Keating, A. E. & Walensky, L. D. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nature Chem. Biol. 6, 595–601 (2010).
Bardwell, P. D. et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J. Immunol. 182, 7482–7489 (2009).
Carrington, E. M. et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc. Natl Acad. Sci. USA 107, 10967–10971 (2010).
Lee, E. F. et al. Discovery and molecular characterization of a Bcl-2-regulated cell death pathway in schistosomes. Proc. Natl Acad. Sci. USA 108, 6999–7003 (2011).
Gavathiotis, E., Reyna, D. E., Bellairs, J. A., Leshchiner, E. S. & Walensky, L. D. Direct and selective small-molecule activation of proapoptotic BAX. Nature Chem. Biol. 8, 639–645 (2012).
Hyman, B. T. & Yuan, J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nature Rev. Neurosci. 13, 395–406 (2012).
Kale, J., Liu, Q., Leber, B. & Andrews, D. W. Shedding light on apoptosis at subcellular membranes. Cell 151, 1179–1184 (2012).
Lovell, J. F. et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135, 1074–1084 (2008).
Shamas-Din, A., Kale, J., Leber, B. & Andrews, D. W. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 5, a008714 (2013).
Karbowski, M., Norris, K. L., Cleland, M. M., Jeong, S. Y. & Youle, R. J. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443, 658–652 (2006).
He, C. et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481, 511–515 (2012).
Chen, Y. B. et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J. Cell Biol. 195, 263–276 (2011).
Verhagen, A. M. et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing inhibitor of apoptosis (IAP) proteins. Cell 102, 43–53 (2000).
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Dai, H., Meng, X. W., Lee, S. H., Schneider, P. A. & Kaufmann, S. H. Context-dependent Bcl-2/Bak interactions regulate lymphoid cell apoptosis. J. Biol. Chem. 284, 18311–18322 (2009).
Lee, E. F. et al. Crystal structure of ABT-737 complexed with Bcl-xL: implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 14, 1711–1713 (2007).
Acknowledgements
P.E.C and G.L contributed equally to this work. The authors thank their colleagues at the Walter and Eliza Hall Institute of Medical Research (WEHI), particularly S. Cory, P. Colman, P. Bouillet, D. Huang, R. Kluck, G. Dewson and D. Westphal, for many stimulating discussions on the issues addressed here, and S. Cory and P. Colman for comments on the manuscript. Their research is supported chiefly by program grant 1016701 and project grants 1025201 and 1025138 from the Australian National Health and Research Council, Australian Research Council (ARC) fellowship FT0992105, funds from the Cancer Council of Victoria and a Center (7417) established by the Leukemia and Lymphoma Society, as well as operational infrastructure grants from the Australian Government (IRISS) and the Victorian State Government (OIS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors had a collaboration with Genentech and Abbott Laboratories (now AbbVie) for cancer drug development.
Glossary
- Sertoli cells
-
Cells that nourish developing sperm cells through the stages of spermatogenesis.
- 'Eat me' signals
-
Surface markers on apoptotic cells, such as phosphatidylserine, that facilitate their recognition and phagocytosis by healthy cells.
- Necroptosis
-
A programmed form of necrosis regulated by receptor-interacting Ser/Thr protein (RIP) kinases.
- Activator BH3-only proteins
-
BH3-only proteins, such as BIM and the truncated form of BID (tBID) that can directly bind and activate BAX or BAK.
- Sensitizer BH3-only proteins
-
BH3-only proteins, such as BAD, that can activate BAX or BAK only indirectly by neutralizing pro-survival BCL-2 family members, thereby preventing them from restraining BAX or BAK.
- Staple
-
A hydrocarbon bridge introduced into a peptide that links amino acids four or seven residues apart to maintain the peptide in a helical conformation, which is thought to convey higher affinity for its target, as well as greater stability and perhaps cell penetration.
- Mitochondrial fission and fusion
-
Mitochondria divide by fission but also continually fuse into tubular networks; thus, their structure is dynamic.
Rights and permissions
About this article
Cite this article
Czabotar, P., Lessene, G., Strasser, A. et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15, 49–63 (2014). https://doi.org/10.1038/nrm3722
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3722
This article is cited by
-
Modulation of apoptosis and Inflammasome activation in chondrocytes: co-regulatory role of Chlorogenic acid
Cell Communication and Signaling (2024)
-
Simultaneous inhibition of FAK and ROS1 synergistically repressed triple-negative breast cancer by upregulating p53 signalling
Biomarker Research (2024)
-
Emerging biomarkers and potential therapeutics of the BCL-2 protein family: the apoptotic and anti-apoptotic context
Egyptian Journal of Medical Human Genetics (2024)
-
Pasteurella multocida activates apoptosis via the FAK-AKT-FOXO1 axis to cause pulmonary integrity loss, bacteremia, and eventually a cytokine storm
Veterinary Research (2024)
-
Prolonged overexpression of PLK4 leads to formation of centriole rosette clusters that are connected via canonical centrosome linker proteins
Scientific Reports (2024)