Key Points
-
Secretory antibodies (SIgs) have long been recognized as immune effectors that protect mucosal epithelia from infection by pathogens.
-
Recent studies have suggested that this important role is only part of the complex biology of SIgs, and that intracellular neutralization of pathogen determinants within epithelial-cell endosomes may be more important in some infection immunity models than immune exclusion (SIg-mediated blocking of pathogen binding or toxin binding to the epithelial surface).
-
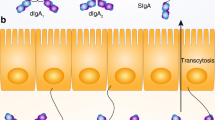
SIgs may also protect the epithelial surface through a phenomenon known as antigen excretion, in which potentially inflammatory antigen in the form of SIg-containing immune complexes is cleared from the subepithelium by the polymeric-immunoglobulin receptor (pIgR).
-
In light of the imperative that is placed on protecting the epithelium from infection in diseases such as HIV/AIDS, and the consequential attempts at mucosal vaccination, it will be important to understand what roles SIgs normally have in maintaining mucosal homeostasis through processes such as T cell regulation and how such processes might be affected by mucosal immunization.
Abstract
The mucosal secretory immune system provides an important primary defence against disease, as studies of humans with mucosal humoral immunodeficiencies suggest that the absence of secretory immunoglobulin A leads to an increase in mucosal infections. However, the infection risks posed do not seem to provide the evolutionary drive to retain constitutive secretion of often 'hard won' protein, suggesting that secretory antibodies may have some other important function (or functions). This Review examines the evidence that secretory antibodies provide an important defence against infection in specific animal models and explores complementary explanations for the evolution of the secretory immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Michetti, P., Mahan, M. J., Slauch, J. M., Mekalanos, J. J. & Neutra, M. R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 60, 1786–1792 (1992).
Johansen, F. E., Braathen, R. & Brandtzaeg, P. Role of J chain in secretory immunoglobulin formation. Scand. J. Immunol. 52, 240–248 (2000).
Vaerman, J. P., Langendries, A., Giffroy, D., Brandtzaeg, P. & Kobayashi, K. Lack of SC/pIgR-mediated epithelial transport of a human polymeric IgA devoid of J chain: in vitro and in vivo studies. Immunology 95, 90–96 (1998).
Hendrickson, B. A. et al. Altered hepatic transport of immunoglobulin A in mice lacking the J chain. J. Exp. Med. 182, 1905–1911 (1995).
Brandtzaeg, P. Mucosal and glandular distribution of immunoglobulin components: differential localization of free and bound SC in secretory epithelial cells. J. Immunol. 112, 1553–1559 (1974).
Brandtzaeg, P. & Prydz, H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature 311, 71–73 (1984).
Lycke, N., Erlandsson, L., Ekman, L., Schon, K. & Leanderson, T. Lack of J chain inhibits the transport of gut IgA and abrogates the development of intestinal antitoxic protection. J. Immunol. 163, 913–919 (1999). A discussion about the role of the J chain in antibody secretion and the requirement for IgA in toxin neutralization.
Shimada, S. et al. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J. Immunol. 163, 5367–5373 (1999).
Uren, T. K. et al. Role of the polymeric Ig receptor in mucosal B cell homeostasis. J. Immunol. 170, 2531–2539 (2003).
Norderhaug, I. N., Johansen, F. E., Schjerven, H. & Brandtzaeg, P. Regulation of the formation and external transport of secretory immunoglobulins. Crit. Rev. Immunol. 19, 481–508 (1999).
Johansen, F. E. et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 190, 915–922 (1999). This work shows that mice that are deficient in pIgR are also deficient for IgA transport, confirming the in vitro models of SIg secretion.
Krajci, P. et al. Molecular cloning of the human transmembrane secretory component (poly-Ig receptor) and its mRNA expression in human tissues. Biochem. Biophys. Res. Commun. 158, 783–789 (1989).
Mostov, K. E., Friedlander, M. & Blobel, G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature 308, 37–43 (1984).
Mostov, K. E., Kraehenbuhl, J. P. & Blobel, G. Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc. Natl Acad. Sci. USA 77, 7257–7261 (1980).
Fallgreen-Gebauer, E. et al. The covalent linkage of secretory component to IgA. structure of sIgA. Biol. Chem. Hoppe Seyler 374, 1023–1028 (1993).
Chintalacharuvu, K. R. et al. Disulfide bond formation between dimeric immunoglobulin A and the polymeric immunoglobulin receptor during hepatic transcytosis. Hepatology 19, 162–173 (1994).
Cerutti, A. & Rescigno, M. The biology of intestinal immunoglobulin A responses. Immunity 28, 740–750 (2008).
Fagarasan, S. Evolution, development, mechanism and function of IgA in the gut. Curr. Opin. Immunol. 20, 170–177 (2008).
Cerutti, A. Location, location, location: B-cell differentiation in the gut lamina propria. Mucosal Immunol. 1, 8–10 (2008).
Honda, K. & Takeda, K. Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2, 187–196 (2009).
Hanson, L. A. & Korotkova, M. The role of breastfeeding in prevention of neonatal infection. Semin. Neonatol. 7, 275–281 (2002).
Shroff, K. E., Meslin, K. & Cebra, J. J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 63, 3904–3913 (1995).
Barone, F., Patel, P., Sanderson, J. D. & Spencer, J. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2, 495–503 (2009).
Yuvaraj, S. et al. Evidence for local expansion of IgA plasma cell precursors in human ileum. J. Immunol. 183, 4871–4878 (2009).
Cazac, B. B. & Roes, J. TGF-β receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 13, 443–451 (2000).
Coffman, R. L., Lebman, D. A. & Shrader, B. Transforming growth factor β specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J. Exp. Med. 170, 1039–1044 (1989).
Ramsay, A. J. et al. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science 264, 561–563 (1994).
Bergqvist, P., Gardby, E., Stensson, A., Bemark, M. & Lycke, N. Y. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J. Immunol. 177, 7772–7783 (2006).
Castigli, E. et al. Impaired IgA class switching in APRIL-deficient mice. Proc. Natl Acad. Sci. USA 101, 3903–3908 (2004).
Castigli, E. et al. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 201, 35–39 (2005).
Litinskiy, M. B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nature Immunol. 3, 822–829 (2002).
Nardelli, B., Moore, P. A., Li, Y. & Hilbert, D. M. B lymphocyte stimulator (BLyS): a therapeutic trichotomy for the treatment of B lymphocyte diseases. Leuk. Lymphoma 43, 1367–1373 (2002).
Roschke, V. et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J. Immunol. 169, 4314–4321 (2002).
Tezuka, H. et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature 448, 929–933 (2007).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Cong, Y., Feng, T., Fujihashi, K., Schoeb, T. R. & Elson, C. O. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl Acad. Sci. USA 106, 19256–19261 (2009). This article demonstrates the role of the intestinal microbiota in shaping both regulation of the mucosal immune system and antibody secretion.
Xu, W. et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nature Immunol. 8, 294–303 (2007).
He, B. et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 (2007).
Mora, J. R. et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160 (2006).
Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nature Immunol. 6, 507–514 (2005).
Nathan, C. Role of iNOS in human host defense. Science 312, 1874–1875 (2006).
Tsuji, M. et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 323, 1488–1492 (2009). The discovery of a pathway for the generation of T H cells from T Reg cells in the gut.
Kroese, F. G. et al. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int. Immunol. 1, 75–84 (1989).
Stoel, M. et al. Restricted IgA repertoire in both B-1 and B-2 cell-derived gut plasmablasts. J. Immunol. 174, 1046–1054 (2005).
Kroese, F. G., de Waard, R. & Bos, N. A. B-1 cells and their reactivity with the murine intestinal microflora. Semin. Immunol. 8, 11–18 (1996).
Kantor, A. B., Stall, A. M., Adams, S., Herzenberg, L. A. & Herzenberg, L. A. Differential development of progenitor activity for three B-cell lineages. Proc. Natl Acad. Sci. USA 89, 3320–3324 (1992).
Ha, S. A. et al. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 203, 2541–2550 (2006).
Macpherson, A. J. et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000). This study identified a T cell-independent secretory antibody that is generated in the gut in response to gut flora.
Macpherson, A. J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
Thurnheer, M. C., Zuercher, A. W., Cebra, J. J. & Bos, N. A. B1 cells contribute to serum IgM, but not to intestinal IgA, production in gnotobiotic Ig allotype chimeric mice. J. Immunol. 170, 4564–4571 (2003).
Boursier, L., Farstad, I. N., Mellembakken, J. R., Brandtzaeg, P. & Spencer, J. IgVH gene analysis suggests that peritoneal B cells do not contribute to the gut immune system in man. Eur. J. Immunol. 32, 2427–2436 (2002).
Brandtzaeg, P., Nilssen, D. E., Rognum, T. O. & Thrane, P. S. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol. Clin. North Am. 20, 397–439 (1991).
Yamanaka, T. et al. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J. Immunol. 170, 816–822 (2003).
Brandtzaeg, P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25, 5467–5484 (2007).
Bos, N. A., Jiang, H. Q. & Cebra, J. J. T cell control of the gut IgA response against commensal bacteria. Gut 48, 762–764 (2001).
Jiang, H. Q. et al. Interactions of commensal gut microbes with subsets of B- and T-cells in the murine host. Vaccine 22, 805–811 (2004).
Notkins, A. L. Polyreactivity of antibody molecules. Trends Immunol. 25, 174–179 (2004).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009). This article and reference 58 discuss how the gut flora shapes the gut immune response and describe the key role of segmented filamentous bacteria in this process.
Bos, N. A. et al. Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect. Immun. 64, 616–623 (1996).
van der Waaij, L. A., Limburg, P. C., Mesander, G. & van der Waaij, D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut 38, 348–354 (1996).
Biesbrock, A. R., Reddy, M. S. & Levine, M. J. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect. Immun. 59, 3492–3497 (1991).
Uren, T. K. et al. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur. J. Immunol. 35, 180–188 (2005).
Allen, J. S., Dougan, G. & Strugnell, R. A. Kinetics of the mucosal antibody secreting cell response and evidence of specific lymphocyte migration to the lung after oral immunisation with attenuated, S. enterica var. typhimurium. FEMS Immunol. Med. Microbiol. 27, 275–281 (2000).
Martinoli, C., Chiavelli, A. & Rescigno, M. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity 27, 975–984 (2007).
Velazquez, C. et al. Giardia lamblia infection induces different secretory and systemic antibody responses in mice. Parasite Immunol. 27, 351–356 (2005).
Iweala, O. I. & Nagler, C. R. Immune privilege in the gut: the establishment and maintenance of non-responsiveness to dietary antigens and commensal flora. Immunol. Rev. 213, 82–100 (2006).
Harriman, G. R. et al. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J. Immunol. 162, 2521–2529 (1999).
Arulanandam, B. P. et al. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J. Immunol. 166, 226–231 (2001).
Zhang, Y. et al. Immunoglobulin A-deficient mice exhibit altered T helper 1-type immune responses but retain mucosal immunity to influenza virus. Immunology 105, 286–294 (2002).
Erlandsson, L., Andersson, K., Sigvardsson, M., Lycke, N. & Leanderson, T. Mice with an inactivated joining chain locus have perturbed IgM secretion. Eur. J. Immunol. 28, 2355–2365 (1998).
Hendrickson, B. A. et al. Lack of association of secretory component with IgA in J chain-deficient mice. J. Immunol. 157, 750–754 (1996).
Sait, L. C. et al. Secretory antibodies reduce systemic antibody responses against the gastrointestinal commensal flora. Int. Immunol. 19, 257–265 (2007).
Wijburg, O. L. et al. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 203, 21–26 (2006). This work elucidates the role of SIg in preventing both the natural transmission and the acquisition of a gastrointestinal pathogen.
Yamazaki, K. et al. Accumulation of intestinal intraepithelial lymphocytes in association with lack of polymeric immunoglobulin receptor. Eur. J. Immunol. 35, 1211–1219 (2005).
Karlsson, M. R., Johansen, F. E., Kahu, H., Macpherson, A. & Brandtzaeg, P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy 65, 561–570 (2009).
Mazanec, M. B., Kaetzel, C. S., Lamm, M. E., Fletcher, D. & Nedrud, J. G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl Acad. Sci. USA 89, 6901–6905 (1992). A demonstration of IgA-mediated intracellular neutralization of pathogens.
Burns, J. W., Siadat-Pajouh, M., Krishnaney, A. A. & Greenberg, H. B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272, 104–107 (1996).
Ruggeri, F. M., Johansen, K., Basile, G., Kraehenbuhl, J. P. & Svensson, L. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J. Virol. 72, 2708–2714 (1998).
Schwartz-Cornil, I., Benureau, Y., Greenberg, H., Hendrickson, B. A. & Cohen, J. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J. Virol. 76, 8110–8117 (2002).
Corthesy, B. et al. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J. Virol. 80, 10692–10699 (2006). A demonstration that intracellular neutralization is more important than immune exclusion for rotavirus.
O'Neal, C. M., Harriman, G. R. & Conner, M. E. Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A. J. Virol. 74, 4102–4109 (2000).
Silvey, K. J., Hutchings, A. B., Vajdy, M., Petzke, M. M. & Neutra, M. R. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer's patches. J. Virol. 75, 10870–10879 (2001).
Wijburg, O. & Strugnell, R. Mucosal immune responses to Escherichia coli and Salmonella infections. In Escherichia coli and Salmonella: cellular and molecular biology (eds Böck, A. et al.) chapter 8.8.12. Ecosal [online] (American Society for Microbiology Press, Washington DC, 2006).
Stager, S. & Muller, N. Giardia lamblia infections in B-cell-deficient transgenic mice. Infect. Immun. 65, 3944–3946 (1997).
Snider, D. P., Skea, D. & Underdown, B. J. Chronic giardiasis in B-cell-deficient mice expressing the xid gene. Infect. Immun. 56, 2838–2842 (1988).
Davids, B. J. et al. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J. Immunol. 177, 6281–6290 (2006).
Langford, T. D. et al. Central importance of immunoglobulin A in host defense against Giardia spp. Infect. Immun. 70, 11–18 (2002).
Phalipon, A. et al. Protection against invasion of the mouse pulmonary epithelium by a monoclonal IgA directed against Shigella flexneri lipopolysaccharide. Ann. NY Acad. Sci. 730, 356–358 (1994).
Michetti, P. et al. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology 107, 915–923 (1994).
Apter, F. M. et al. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect. Immun. 61, 5279–5285 (1993).
Czinn, S. J., Cai, A. & Nedrud, J. G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine 11, 637–642 (1993).
Cunningham, K. A. et al. Poly-immunoglobulin receptor-mediated transport of IgA into the male genital tract is important for clearance of Chlamydia muridarum infection. Am. J. Reprod. Immunol. 60, 405–414 (2008).
Sun, K., Johansen, F. E., Eckmann, L. & Metzger, D. W. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J. Immunol. 173, 4576–4581 (2004).
Way, S. S., Borczuk, A. C. & Goldberg, M. B. Adaptive immune response to Shigella flexneri 2a cydC in immunocompetent mice and mice lacking immunoglobulin A. Infect. Immun. 67, 2001–2004 (1999).
Jones, B. D. & Falkow, S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14, 533–561 (1996).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2, 361–367 (2001).
Vazquez-Torres, A. et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401, 804–808 (1999).
Tjarnlund, A. et al. Polymeric IgR knockout mice are more susceptible to mycobacterial infections in the respiratory tract than wild-type mice. Int. Immunol. 18, 807–816 (2006).
Rodriguez, A. et al. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine 23, 2565–2572 (2005).
Brandtzaeg, P., Fjellanger, I. & Gjeruldsen, S. T. Adsorption of immunolgobulin A onto oral bacteria in vivo. J. Bacteriol. 96, 242–249 (1968).
Kaetzel, C. S., Robinson, J. K., Chintalacharuvu, K. R., Vaerman, J. P. & Lamm, M. E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc. Natl Acad. Sci. USA 88, 8796–8800 (1991). A study showing antigen excretion mediated by pIgR.
Yan, H., Lamm, M. E., Bjorling, E. & Huang, Y. T. Multiple functions of immunoglobulin A in mucosal defense against viruses: an in vitro measles virus model. J. Virol. 76, 10972–10979 (2002).
Wright, A., Lamm, M. E. & Huang, Y. T. Excretion of human immunodeficiency virus type 1 through polarized epithelium by immunoglobulin A. J. Virol. 82, 11526–11535 (2008).
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77, 11708–11717 (2003).
Arsenescu, R. et al. Signature biomarkers in Crohn's disease: toward a molecular classification. Mucosal Immunol. 1, 399–411 (2008).
Murthy, A. K., Dubose, C. N., Banas, J. A., Coalson, J. J. & Arulanandam, B. P. Contribution of polymeric immunoglobulin receptor to regulation of intestinal inflammation in dextran sulfate sodium-induced colitis. J. Gastroenterol. Hepatol. 21, 1372–1380 (2006).
Hibi, T., Ogata, H. & Sakuraba, A. Animal models of inflammatory bowel disease. J. Gastroenterol. 37, 409–417 (2002).
Takenouchi-Ohkubo, N. et al. Role of nuclear factor-κB in the expression by tumor necrosis factor-α of the human polymeric immunoglobulin receptor (plgR) gene. Immunogenetics 51, 289–295 (2000).
Schjerven, H., Brandtzaeg, P. & Johansen, F. E. A novel NF-κB/Rel site in intron 1 cooperates with proximal promoter elements to mediate TNF-α-induced transcription of the human polymeric Ig receptor. J. Immunol. 167, 6412–6420 (2001).
Hempen, P. M. et al. Transcriptional regulation of the human polymeric Ig receptor gene: analysis of basal promoter elements. J. Immunol. 169, 1912–1921 (2002).
Hooper, L. V. et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001).
Pal, K., Kaetzel, C. S., Brundage, K., Cunningham, C. A. & Cuff, C. F. Regulation of polymeric immunoglobulin receptor expression by reovirus. J. Gen. Virol. 86, 2347–2357 (2005).
Schneeman, T. A. et al. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J. Immunol. 175, 376–384 (2005).
Muir, A. et al. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell. Mol. Biol. 30, 777–783 (2004).
Agarwal, S. & Mayer, L. Pathogenesis and treatment of gastrointestinal disease in antibody deficiency syndromes. J. Allergy Clin. Immunol. 124, 658–664 (2009).
Cunningham-Rundles, C. & Bodian, C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin. Immunol. 92, 34–48 (1999).
Royle, L. et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 278, 20140–20153 (2003).
Dallas, S. D. & Rolfe, R. D. Binding of Clostridium difficile toxin A to human milk secretory component. J. Med. Microbiol. 47, 879–888 (1998).
Perrier, C., Sprenger, N. & Corthesy, B. Glycans on secretory component participate in innate protection against mucosal pathogens. J. Biol. Chem. 281, 14280–14287 (2006).
Phalipon, A. et al. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17, 107–115 (2002).
Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350, 423–426 (1991).
Brundler, M. A. et al. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur. J. Immunol. 26, 2257–2262 (1996).
Macpherson, A. J. et al. IgA production without μ or δ chain expression in developing B cells. Nature Immunol. 2, 625–631 (2001). This investigation demonstrates that SIg production can occur in μMT−/− mice (mice deficient for the Igμ transmembrane tail exons), revealing a separate lineage of SIg production.
McCoy, K. D. et al. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe 4, 362–373 (2008).
Maaser, C. et al. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 72, 3315–3324 (2004).
Simmons, C. P. et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 71, 5077–5086 (2003).
Gibson, A. et al. Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells. J. Cell Biol. 143, 81–94 (1998).
Lindh, E. Increased risistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J. Immunol. 114, 284–286 (1975).
Underdown, B. J. & Dorrington, K. J. Studies on the structural and conformational basis for the relative resistance of serum and secretory immunoglobulin A to proteolysis. J. Immunol. 112, 949–959 (1974).
Acknowledgements
The authors thank all students and staff from the University of Melbourne, Australia, who have contributed to the SIg projects. O.W. is a Career Development Award recipient of the National Health and Medical Research Council (Australia). The authors apologize to those who have contributed to this field but could not be cited.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome Project
Salmonella enterica subsp. enterica serovar Typhimurium
FURTHER INFORMATION
Glossary
- Backpack tumour
-
Antibody-secreting tumour that is induced by the transfer of antibody-secreting hybridomas to naive animals through subcutaneous injection of the hybridomas in an area of the back.
- Antibody avidity
-
The sum of the affinities of individual Fab–epitope interactions. Dimeric antibodies will have a higher avidity than monomeric antibodies. Antigens with repeating identical epitopes are bound with higher avidity than antigens that carry a single epitope.
- Columnar epithelial cell
-
Differentiated epithelial cell that lines mucosal surfaces, with a column-like appearance and an elongated nucleus. These cells have a lumen-facing ('apical') surface and a tissue facing ('basolateral') surface.
- Class switching
-
Occurs when the activated B cell rearranges the genes encoding the constant region of the antibody molecule that is produced; this rearrangement is usually dependent on cytokines produced by T cells. Hence, a single B cell may produce daughter cells that produce different isotypes (for example, IgG and IgE) of the same antibody specificity. Also known as isotype switching.
- Monoassociation
-
The introduction of a single species, usually a gut flora species, into a germ-free animal.
- Complement
-
Cascade of more than 20 serum glycoproteins that interact to affect some aspects of humoral immunity (for example, lysis of bacteria and opsonization) and which may also drive inflammatory processes such as neutrophil recruitment.
- Peyer's patch
-
Region of differentiated lymphoid tissue found in the lamina propria of the small intestine, containing B cell-rich germinal centres separated by regions containing T cells. The so-called 'dome' epithelium that overlies the Peyer's patch is rich in 'microfold' (M) cells, which sample antigen from the gut for presentation in secondary lymphoid tissues.
- B cell antigen-presenting function
-
In addition to producing antibodies, B cells also express major histocompatiblity complex (MHC) class II molecules and can act as an antigen-presenting cell to CD4+ T cells. In mice such as the μMT/−/− mouse, altered protection might be ascribed to either loss of antibodies or failure of B cells to present antigen to T cells.
Rights and permissions
About this article
Cite this article
Strugnell, R., Wijburg, O. The role of secretory antibodies in infection immunity. Nat Rev Microbiol 8, 656–667 (2010). https://doi.org/10.1038/nrmicro2384
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2384
This article is cited by
-
Intestinal mucus components and secretion mechanisms: what we do and do not know
Experimental & Molecular Medicine (2023)
-
SARS-CoV-2 specific sIgA in saliva increases after disease-related video stimulation
Scientific Reports (2023)
-
Immunoregulatory nanomedicine for respiratory infections
Nature Reviews Bioengineering (2023)
-
Issues for patchy tissues: defining roles for gut-associated lymphoid tissue in neurodevelopment and disease
Journal of Neural Transmission (2023)
-
Self-perceived Mate Value is Predicted by Biological and self-reported Indices of Health in Young Adults
Adaptive Human Behavior and Physiology (2023)