Key Points
-
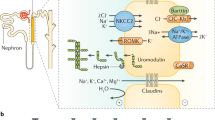
Uromodulin — the most abundant urinary protein — is exclusively produced by renal epithelial cells; in the tubular lumen uromodulin forms high-molecular weight filaments that constitute the matrix of hyaline casts
-
Important functions of uromodulin include regulation of ion transport in the thick ascending limb, immunomodulation and protection against urinary tract infections and kidney stones
-
Levels of uromodulin in the urine and in the blood, where it is present in lower amounts, are valuable biomarkers for tubular mass and renal function
-
Rare mutations in UMOD cause autosomal dominant tubulointerstitial kidney disease; these mutations lead to retention of mutant uromodulin in the endoplasmic reticulum of tubular cells, tubulointerstitial damage and decreased levels of urinary uromodulin
-
Common variants in the UMOD promoter are associated with risk of chronic kidney disease (CKD) and hypertension; the unusually high prevalence of UMOD risk alleles suggests pathogen-driven selective pressure
-
UMOD represents a paradigm as a continuum of genetic disease risk, from rare mutations in Mendelian disease to common variants associated with complex traits including CKD and hypertension
Abstract
Uromodulin (also known as Tamm-Horsfall protein) is exclusively produced in the kidney and is the most abundant protein in normal urine. The function of uromodulin remains elusive, but the available data suggest that this protein might regulate salt transport, protect against urinary tract infection and kidney stones, and have roles in kidney injury and innate immunity. Interest in uromodulin was boosted by genetic studies that reported involvement of the UMOD gene, which encodes uromodulin, in a spectrum of rare and common kidney diseases. Rare mutations in UMOD cause autosomal dominant tubulointerstitial kidney disease (ADTKD), which leads to chronic kidney disease (CKD). Moreover, genome-wide association studies have identified common variants in UMOD that are strongly associated with risk of CKD and also with hypertension and kidney stones in the general population. These findings have opened up a new field of kidney research. In this Review we summarize biochemical, physiological, genetic and pathological insights into the roles of uromodulin; the mechanisms by which UMOD mutations cause ADTKD, and the association of common UMOD variants with complex disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rovida, C. L. Conclusione degli studi intorno all'origine istologica dei cilindri dell'urina. Riv. Clin. Bologna 2a, 303–306 (1873).
Tamm, I. & Horsfall, F. L. Jr. Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc. Soc. Exp. Biol. Med. 74, 106–108 (1950).
Wenk, R. E., Bhagavan, B. S. & Rudert, J. Tamm-Horsfall uromucoprotein and the pathogenesis of casts, reflux nephropathy, and nephritides. Pathobiol. Annu. 11, 229–257 (1981).
McKenzie, J. K. & McQueen, E. G. Immunofluorescent localization of Tamm-Horsfall mucoprotein in human kidney. J. Clin. Pathol. 22, 334–339 (1969).
McQueen, E. G. The nature of urinary casts. J. Clin. Pathol. 15, 367–373 (1962).
Muchmore, A. V. & Decker, J. M. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science 229, 479–481 (1985).
Pennica, D. et al. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science 236, 83–88 (1987). Identification of uromodulin as Tamm-Horsfall glycoprotein and of its key properties, including glycosylation and kidney-specific synthesis.
Rampoldi, L., Scolari, F., Amoroso, A., Ghiggeri, G. & Devuyst, O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 80, 338–347 (2011).
Hart, T. C. et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J. Med. Genet. 39, 882–892 (2002). First identification of mutations in UMOD as the cause of the rare disorders medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy.
Dahan, K. et al. A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. J. Am. Soc. Nephrol. 14, 2883–2893 (2003). Demonstration that UMOD mutations are associated with intracellular retention of uromodulin and reduced urinary uromodulin levels.
Kottgen, A. et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 41, 712–717 (2009). The first GWAS to demonstrate an association of UMOD variants with eGFR and CKD in the general population.
Pattaro, C. et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat. Commun. 7, 10023 (2016). The largest meta-analysis for eGFR and CKD reported so far, demonstrating the predominant size effect of the UMOD locus.
Trudu, M. et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat. Med. 19, 1655–1660 (2013). Demonstration of the biological effect of UMOD variants in association with salt-sensitive hypertension and kidney damage.
Olden, M. et al. Common variants in UMOD associate with urinary uromodulin levels: a meta-analysis. J. Am. Soc. Nephrol. 25, 1869–1882 (2014). First meta-GWAS demonstrating the association of UMOD variants with urinary levels of uromodulin.
Hession, C. et al. Uromodulin (Tamm-Horsfall glycoprotein): a renal ligand for lymphokines. Science 237, 1479–1484 (1987).
Prasadan, K. et al. Nucleotide sequence and peptide motifs of mouse uromodulin (Tamm-Horsfall protein)—the most abundant protein in mammalian urine. Biochim. Biophys. Acta 1260, 328–332 (1995).
Rindler, M. J., Naik, S. S., Li, N., Hoops, T. C. & Peraldi, M. N. Uromodulin (Tamm-Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J. Biol. Chem. 265, 20784–20789 (1990).
Bokhove, M. et al. A structured interdomain linker directs self-polymerization of human uromodulin. Proc. Natl Acad. Sci. USA 113, 1552–1557 (2016). Crystal structure of uromodulin suggesting the possible mechanism of polymerization.
Jovine, L., Qi, H., Williams, Z., Litscher, E. & Wassarman, P. M. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat. Cell Biol. 4, 457–461 (2002).
Campbell, I. D. & Bork, P. Epidermal growth factor-like modules. Curr. Opin. Struc Biol. 3, 385–392 (1993).
Grant, A. M. & Neuberger, A. The turnover rate of rabbit urinary Tamm-Horsfall glycoprotein. Biochem. J. 136, 659–668 (1973).
Fletcher, A. P., Neuberger, A. & Ratcliffe, W. A. Tamm-Horsfall urinary glycoprotein. The chemical composition. Biochem. J. 120, 417–424 (1970).
Huang, Z. Q., Kirk, K. A., Connelly, K. G. & Sanders, P. W. Bence Jones proteins bind to a common peptide segment of Tamm-Horsfall glycoprotein to promote heterotypic aggregation. J. Clin. Invest. 92, 2975–2983 (1993).
Serafini-Cessi, F., Malagolini, N., Hoops, T. C. & Rindler, M. J. Biosynthesis and oligosaccharide processing of human Tamm-Horsfall glycoprotein permanently expressed in HeLa cells. Biochem. Biophys. Res. Commun. 194, 784–790 (1993).
Malagolini, N., Cavallone, D. & Serafini-Cessi, F. Intracellular transport, cell-surface exposure and release of recombinant Tamm-Horsfall glycoprotein. Kidney Int. 52, 1340–1350 (1997).
Rampoldi, L. et al. Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum. Mol. Genet. 12, 3369–3384 (2003).
Bernascone, I. et al. Defective intracellular trafficking of uromodulin mutant isoforms. Traffic 7, 1567–1579 (2006).
van Rooijen, J. J., Voskamp, A. F., Kamerling, J. P. & Vliegenthart, J. F. Glycosylation sites and site-specific glycosylation in human Tamm-Horsfall glycoprotein. Glycobiology 9, 21–30 (1999).
Pesce, A. J. et al. Renal tubular interactions of proteins. Clin. Biochem. 13, 209–215 (1980).
Donald, A. S., Yates, A. D., Soh, C. P., Morgan, W. T. & Watkins, W. M. A blood group Sda-active pentasaccharide isolated from Tamm-Horsfall urinary glycoprotein. Biochem. Biophys. Res. Commun. 115, 625–631 (1983).
Serafini-Cessi, F., Malagolini, N. & Cavallone, D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am. J. Kidney Dis. 42, 658–676 (2003).
Easton, R. L., Patankar, M. S., Clark, G. F., Morris, H. R. & Dell, A. Pregnancy-associated changes in the glycosylation of Tamm-Horsfall glycoprotein. Expression of sialyl Lewis(x) sequences on core 2 type O-glycans derived from uromodulin. J. Biol. Chem. 275, 21928–21938 (2000).
Simons, K. & Ikonen, E. Functional rafts in cell membranes. Nature 387, 569–572 (1997).
Welker, P. et al. Role of lipid rafts in membrane delivery of renal epithelial Na+-K+-ATPase, thick ascending limb. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1328–R1337 (2007).
Brown, D. A., Crise, B. & Rose, J. K. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science 245, 1499–1501 (1989).
Benting, J. H., Rietveld, A. G. & Simons, K. N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells. J. Cell Biol. 146, 313–320 (1999).
Schaeffer, C., Santambrogio, S., Perucca, S., Casari, G. & Rampoldi, L. Analysis of uromodulin polymerization provides new insights into the mechanisms regulating ZP domain-mediated protein assembly. Mol. Biol. Cell 20, 589–599 (2009).
Bachmann, S., Dawnay, A. B., Bouby, N. & Bankir, L. Tamm-Horsfall protein excretion during chronic alterations in urinary concentration and protein intake in the rat. Ren Physiol. Biochem. 14, 236–245 (1991).
Goodall, A. A. & Marshall, R. D. Effects of freezing on the estimated amounts of Tamm—Horsfall glycoprotein in urine, as determined by radioimmunoassay. Biochem. J. 189, 533–539 (1980).
Maxfield, M. Molecular forms of human urinary mucoprotein present under physiological conditions. Biochim. Biophys. Acta 49, 548–558 (1961).
Porter, K. R. & Tamm, I. Direct visualization of a mucoprotein component of urine. J. Biol. Chem. 212, 135–140 (1955).
Wiggins, R. C. Uromucoid (Tamm-Horsfall glycoprotein) forms different polymeric arrangements on a filter surface under different physicochemical conditions. Clin. Chim. Acta 162, 329–340 (1987).
Santambrogio, S. et al. Urinary uromodulin carries an intact ZP domain generated by a conserved C-terminal proteolytic cleavage. Biochem. Biophys. Res. Commun. 370, 410–413 (2008).
Brunati, M. et al. The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. eLife 4, e08887 (2015). Identification of hepsin as the protease responsible for the release of uromodulin into the tubular lumen.
Grant, A. M. & Neuberger, A. The development of a radioimmunoassay for the measurement of urinary Tamm-Horsfall glycoprotein in the presence of sodium dodecyl sulphate. Clin. Sci. 44, 163–179 (1973).
Youhanna, S. et al. Determination of uromodulin in human urine: influence of storage and processing. Nephrol. Dial Transplant 29, 136–145 (2014).
Shihabi, Z. K., Hinsdale, M. E. & Bleyer, A. J. Analysis of Tamm-Horsfall protein by high-performance liquid chromatography with native fluorescence. J. Chromatogr. A 1027, 161–166 (2004).
Hammond, T. G. et al. Development and characterization of a pseudo multiple reaction monitoring method for the quantification of human uromodulin in urine. Bioanalysis 8, 1279–1296 (2016).
Scherberich, J. E. et al. Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrol. Dial Transplant. http://dx.doi.org/10.1093/ndt/gfw422 (2017).
Ying, W. Z. & Sanders, P. W. Dietary salt regulates expression of Tamm-Horsfall glycoprotein in rats. Kidney Int. 54, 1150–1156 (1998).
Torffvit, O., Melander, O. & Hulten, U. L. Urinary excretion rate of Tamm-Horsfall protein is related to salt intake in humans. Nephron Physiol. 97, 31–36 (2004).
Padmanabhan, S. et al. Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet. 6, e1001177 (2010). Demonstration of the association of UMOD variants with hypertension.
Ecelbarger, C. A. et al. Localization and regulation of the rat renal Na+-K+-2Cl- cotransporter BSC-1. Am. J. Physiol. 271, F619–F628 (1996).
Schmitt, R., Kahl, T., Mutig, K. & Bachmann, S. Selectively reduced expression of thick ascending limb Tamm-Horsfall protein in hypothyroid kidneys. Histochem. Cell Biol. 121, 319–327 (2004).
Pook, M. A., Jeremiah, S., Scheinman, S. J., Povey, S. & Thakker, R. V. Localization of the Tamm-Horsfall glycoprotein (uromodulin) gene to chromosome 16p12.3-16p13.11. Ann. Hum. Genet. 57, 285–290 (1993).
Zhu, X. et al. Isolation of mouse THP gene promoter and demonstration of its kidney-specific activity in transgenic mice. Am. J. Physiol. Renal Physiol. 282, F608–F617 (2002).
Uhlen, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Lee, J. W., Chou, C. L. & Knepper, M. A. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J. Am. Soc. Nephrol. 26, 2669–2677 (2015).
Cheval, L. et al. Atlas of gene expression in the mouse kidney: new features of glomerular parietal cells. Physiol. Genom. 43, 161–173 (2011).
Bachmann, S., Metzger, R. & Bunnemann, B. Tamm-Horsfall protein-mRNA synthesis is localized to the thick ascending limb of Henle's loop in rat kidney. Histochemistry 94, 517–523 (1990).
de Baaij, J. H. et al. Elucidation of the distal convoluted tubule transcriptome identifies new candidate genes involved in renal Mg2+ handling. Am. J. Physiol. Renal Physiol. 305, F1563–F1573 (2013).
Sikri, K. L., Foster, C. L., MacHugh, N. & Marshall, R. D. Localization of Tamm-Horsfall glycoprotein in the human kidney using immuno-fluorescence and immuno-electron microscopical techniques. J. Anat. 132, 597–605 (1981).
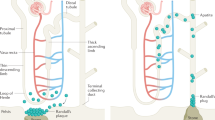
El-Achkar, T. M. et al. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am. J. Physiol. Renal Physiol. 304, F1066–F1075 (2013).
Kumar, S. & Muchmore, A. Tamm-Horsfall protein—uromodulin (1950–1990). Kidney Int. 37, 1395–1401 (1990).
Brunskill, E. W. et al. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev. Cell 15, 781–791 (2008).
Sikri, K. L., Foster, C. L., Alexander, D. P. & Marshall, R. D. Localization of Tamm-Horsfall glycoprotein in the fetal and neonatal hamster kidney as demonstrated by immunofluorescence and immunoelectron microscopical techniques. Biol. Neonate 39, 305–312 (1981).
Zimmerhackl, L. B. et al. Tamm-Horsfall protein as a marker of tubular maturation. Pediatr. Nephrol. 10, 448–452 (1996).
DeFreitas, M. J. et al. Longitudinal patterns of urine biomarkers in infants across gestational ages. Pediatr. Nephrol. 31, 1179–1188 (2016).
Kim, H. T., Song, I. Y. & Piedrahita, J. Kidney-specific activity of the bovine uromodulin promoter. Transgen. Res. 12, 191–201 (2003).
Zbikowska, H. M. et al. The use of the uromodulin promoter to target production of recombinant proteins into urine of transgenic animals. Transgen. Res. 11, 425–435 (2002).
Stricklett, P. K., Taylor, D., Nelson, R. D. & Kohan, D. E. Thick ascending limb-specific expression of Cre recombinase. Am. J. Physiol. Renal Physiol. 285, F33–39 (2003).
Srivastava, R., Micanovic, R., El-Achkar, T. M. & Janga, S. C. An intricate network of conserved DNA upstream motifs and associated transcription factors regulate the expression of uromodulin gene. J. Urol. 192, 981–989 (2014).
Gresh, L. et al. A transcriptional network in polycystic kidney disease. EMBO J. 23, 1657–1668 (2004).
Rosenbloom, K. R. et al. The UCSC Genome Browser database: 2015 update. Nucleic Acids Res. 43, D670–D681 (2015).
Badgett, A. & Kumar, S. Phylogeny of Tamm-Horsfall protein. Urol. Int. 61, 72–75 (1998).
Wallace, A. C. & Nairn, R. C. Tamm-Horsfall protein in kidneys of human embryos and foreign species. Pathology 3, 303–310 (1971).
Howie, A. J., Lote, C. J., Cunningham, A. A., Zaccone, G. & Fasulo, S. Distribution of immunoreactive Tamm-Horsfall protein in various species in the vertebrate classes. Cell Tissue Res. 274, 15–19 (1993).
Fukuoka, S., Freedman, S. D., Yu, H., Sukhatme, V. P. & Scheele, G. A. GP-2/THP gene family encodes self-binding glycosylphosphatidylinositol-anchored proteins in apical secretory compartments of pancreas and kidney. Proc. Natl Acad. Sci. USA 89, 1189–1193 (1992).
Ronco, P. et al. Physiopathologic aspects of Tamm-Horsfall protein: a phylogenetically conserved marker of the thick ascending limb of Henle's loop. Adv. Nephrol. Necker Hosp. 16, 231–249 (1987).
Kondo, Y. et al. Phylogenetic, ontogenetic, and pathological aspects of the urine-concentrating mechanism. Clin. Exp. Nephrol. 10, 165–174 (2006).
Mutig, K. et al. Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J. Biol. Chem. 286, 30200–30210 (2011).
Renigunta, A. et al. Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function. J. Biol. Chem. 286, 2224–2235 (2011).
Bachmann, S. et al. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am. J. Physiol. Renal Physiol. 288, F559–F567 (2005). Study of the effects of lack of uromodulin on murine kidney physiology.
Graham, L. A. et al. Validation of uromodulin as a candidate gene for human essential hypertension. Hypertension 63, 551–558 (2014).
Coe, F. L., Evan, A. & Worcester, E. Pathophysiology-based treatment of idiopathic calcium kidney stones. Clin. J. Am. Soc. Nephrol. 6, 2083–2092 (2011).
Serafini-Cessi, F., Monti, A. & Cavallone, D. N-Glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconj J. 22, 383–394 (2005). Demonstration that binding of uromodulin to uropathogenic E. coli is largely mediated by protein glycans.
Gokhale, J. A., Glenton, P. A. & Khan, S. R. Characterization of Tamm-Horsfall protein in a rat nephrolithiasis model. J. Urol. 166, 1492–1497 (2001).
Hallson, P. C., Choong, S. K., Kasidas, G. P. & Samuell, C. T. Effects of Tamm-Horsfall protein with normal and reduced sialic acid content upon the crystallization of calcium phosphate and calcium oxalate in human urine. Br. J. Urol. 80, 533–538 (1997).
Liu, Y. et al. Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm-Horsfall protein. Am. J. Physiol. Renal Physiol. 299, F469–478 (2010).
Mo, L. et al. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 66, 1159–1166 (2004). Increased incidence of kidney stones in mice lacking uromodulin.
Coe, F. L., Worcester, E. M. & Evan, A. P. Idiopathic hypercalciuria and formation of calcium renal stones. Nat. Rev. Nephrol. 12, 519–533 (2016).
Wolf, M. T., Wu, X. R. & Huang, C. L. Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int. 84, 130–137 (2013).
Gudbjartsson, D. F. et al. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet. 6, e1001039 (2010).
Mo, L. et al. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am. J. Physiol. Renal Physiol. 286, F795–F802 (2004).
Bates, J. M. et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 65, 791–797 (2004). Increased susceptibility to urinary tract infections by type 1-fimbriated E. coli in mice lacking uromodulin.
Pak, J., Pu, Y., Zhang, Z. T., Hasty, D. L. & Wu, X. R. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J. Biol. Chem. 276, 9924–9930 (2001).
Garimella, P. S. et al. Urinary uromodulin and risk of urinary tract infections: the Cardiovascular Health Study. Am. J. Kidney Dis. 69, 744–751 (2016).
Ghirotto, S. et al. The uromodulin gene locus shows evidence of pathogen adaptation through human evolution. J. Am. Soc. Nephrol. 27, 2983–2996 (2016). Identification of pathogen-driven selection at the UMOD locus, which could explain the high prevalence of the deleterious allele in most populations.
Rhodes, D. C., Hinsman, E. J. & Rhodes, J. A. Tamm-Horsfall glycoprotein binds IgG with high affinity. Kidney Int. 44, 1014–1021 (1993).
Muchmore, A. V., Shifrin, S. & Decker, J. M. In vitro evidence that carbohydrate moieties derived from uromodulin, an 85,000 dalton immunosuppressive glycoprotein isolated from human pregnancy urine, are immunosuppressive in the absence of intact protein. J. Immunol. 138, 2547–2553 (1987).
Springer, G. F., Schwick, H. G. & Fletcher, M. A. The relationship of the influenza virus inhibitory activity of glycoproteins to their molecular size and sialic acid content. Proc. Natl Acad. Sci. USA 64, 634–641 (1969).
El-Achkar, T. M. et al. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am. J. Physiol. Renal Physiol. 300, F999–1007 (2011).
El-Achkar, T. M. et al. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am. J. Physiol. Renal Physiol. 295, F534–F544 (2008).
Micanovic, R. et al. Tamm-Horsfall protein regulates granulopoiesis and systemic neutrophil homeostasis. J. Am. Soc. Nephrol. 26, 2172–2182 (2015).
Saemann, M. D. et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J. Clin. Invest. 115, 468–475 (2005). Link between uromodulin and innate and adaptive immunity in the urinary tract.
Darisipudi, M. N. et al. Uromodulin triggers IL-1β-dependent innate immunity via the NLRP3 inflammasome. J. Am. Soc. Nephrol. 23, 1783–1789 (2012). Uromodulin can behave as a danger signal and promote inflammation in the interstititum via activation of the inflammasome.
Thomas, D. B., Davies, M., Peters, J. R. & Williams, J. D. Tamm Horsfall protein binds to a single class of carbohydrate specific receptors on human neutrophils. Kidney Int. 44, 423–429 (1993).
Wimmer, T., Cohen, G., Saemann, M. D. & Horl, W. H. Effects of Tamm-Horsfall protein on polymorphonuclear leukocyte function. Nephrol. Dial Transplant 19, 2192–2197 (2004).
Schmid, M. et al. Uromodulin facilitates neutrophil migration across renal epithelial monolayers. Cell Physiol. Biochem. 26, 311–318 (2010).
Sanders, P. W., Booker, B. B., Bishop, J. B. & Cheung, H. C. Mechanisms of intranephronal proteinaceous cast formation by low molecular weight proteins. J. Clin. Invest. 85, 570–576 (1990).
Hutchison, C. A. et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat. Rev. Nephrol. 8, 43–51 (2011).
Start, D. A., Silva, F. G., Davis, L. D., D'Agati, V. & Pirani, C. L. Myeloma cast nephropathy: immunohistochemical and lectin studies. Mod. Pathol. 1, 336–347 (1988).
Ying, W. Z., Allen, C. E., Curtis, L. M., Aaron, K. J. & Sanders, P. W. Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. J. Clin. Invest. 122, 1777–1785 (2012).
Sanders, P. W. & Booker, B. B. Pathobiology of cast nephropathy from human Bence Jones proteins. J. Clin. Invest. 89, 630–639 (1992).
Huang, Z. Q. & Sanders, P. W. Localization of a single binding site for immunoglobulin light chains on human Tamm-Horsfall glycoprotein. J. Clin. Invest. 99, 732–736 (1997).
Ying, W. Z. & Sanders, P. W. Mapping the binding domain of immunoglobulin light chains for Tamm-Horsfall protein. Am. J. Pathol. 158, 1859–1866 (2001).
Askenazi, D. J. et al. Acute kidney injury urine biomarkers in very low-birth-weight infants. Clin. J. Am. Soc. Nephrol. 11, 1527–1535 (2016).
Schroter, J., Timmermans, G., Seyberth, H. W., Greven, J. & Bachmann, S. Marked reduction of Tamm-Horsfall protein synthesis in hyperprostaglandin E-syndrome. Kidney Int. 44, 401–410 (1993).
Lynn, K. L. & Marshall, R. D. Excretion of Tamm-Horsfall glycoprotein in renal disease. Clin. Nephrol. 22, 253–257 (1984).
Thornley, C., Dawnay, A. & Cattell, W. R. Human Tamm-Horsfall glycoprotein: urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin. Sci. (Lond.) 68, 529–535 (1985). Demonstration of a correlation between plasma and urine levels of uromodulin; differences in concentrations; and influence by CKD.
Matafora, V. et al. Early markers of Fabry disease revealed by proteomics. Mol. Biosyst 11, 1543–1551 (2015).
Tsai, C. Y., Wu, T. H., Yu, C. L., Lu, J. Y. & Tsai, Y. Y. Increased excretions of β2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron 85, 207–214 (2000).
Kistler, A. D. et al. Identification of a unique urinary biomarker profile in patients with autosomal dominant polycystic kidney disease. Kidney Int. 76, 89–96 (2009).
Rasch, R., Torffvit, O., Bachmann, S., Jensen, P. K. & Jacobsen, N. O. Tamm-Horsfall glycoprotein in streptozotocin diabetic rats: a study of kidney in situ hybridization, immunohistochemistry, and urinary excretion. Diabetologia 38, 525–535 (1995).
Bernard, A. M., Ouled, A. A., Lauwerys, R. R., Lambert, A. & Vandeleene, B. Pronounced decrease of Tamm-Horsfall proteinuria in diabetics. Clin. Chem. 33, 1264 (1987).
Torffvit, O. & Agardh, C. D. Tubular secretion of Tamm-Horsfall protein is decreased in type 1 (insulin-dependent) diabetic patients with diabetic nephropathy. Nephron 65, 227–231 (1993).
Pruijm, M. et al. Associations of urinary uromodulin with clinical characteristics and markers of tubular function in the general population. Clin. J. Am. Soc. Nephrol. 11, 70–80 (2016). Population-based study demonstrating that urinary uromodulin levels correlate with functioning nephron mass and tubular function parameters.
Troyanov, S. et al. Clinical, genetic, and urinary factors associated with uromodulin excretion. Clin. J. Am. Soc. Nephrol. 11, 62–69 (2016).
Ledo, N. et al. Functional genomic annotation of genetic risk loci highlights inflammation and epithelial biology networks in CKD. J. Am. Soc. Nephrol. 26, 692–714 (2015).
Garimella, P. S. et al. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int. 88, 1126–1134 (2015).
Yoshida, T. et al. Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int. 61, 1646–1654 (2002).
Garimella, P. S. et al. Association of preoperative urinary uromodulin with AKI after cardiac surgery. Clin. J. Am. Soc. Nephrol. 12, 10–18 (2017).
Ganter, K., Bongartz, D. & Hesse, A. Tamm-Horsfall protein excretion and its relation to citrate in urine of stone-forming patients. Urology 53, 492–495 (1999).
Pourmand, G. et al. Comparison of urinary proteins in calcium stone formers and healthy individuals: a case-control study. Urol. Int. 76, 163–168 (2006).
Knorle, R. et al. Tamm-Horsfall glycoprotein: role in inhibition and promotion of renal calcium oxalate stone formation studied with Fourier-transform infrared spectroscopy. Clin. Chem. 40, 1739–1743 (1994).
Trewick, A. L. & Rumsby, G. Isoelectric focusing of native urinary uromodulin (Tamm-Horsfall protein) shows no physicochemical differences between stone formers and non-stone formers. Urol. Res. 27, 250–254 (1999).
Dawnay, A. B. & Cattell, W. R. Serum Tamm-Horsfall glycoprotein levels in health and in renal disease. Clin. Nephrol. 15, 5–8 (1981).
Steubl, D. et al. Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Med. (Baltimore) 95, e3011 (2016). Demonstration of the correlation of serum uromodulin levels with renal function.
Fedak, D. et al. Serum uromodulin concentrations correlate with glomerular filtration rate in patients with chronic kidney disease. Pol. Arch. Med. Wewn 126, 995–1004 (2016).
Risch, L. et al. The serum uromodulin level is associated with kidney function. Clin. Chem. Lab Med. 52, 1755–1761 (2014).
Steubl, D. et al. Serum uromodulin predicts graft failure in renal transplant recipients. Biomarkers 22, 171–177 (2017).
Prajczer, S. et al. Evidence for a role of uromodulin in chronic kidney disease progression. Nephrol. Dial Transplant 25, 1896–1903 (2010).
Alfaham, M., Peters, T. J., Meyrick, S., Avis, P. & Verrier Jones, K. Serum Tamm-Horsfall protein levels in childhood: relationship with age and glomerular filtration rate. Nephron 52, 216–221 (1989).
Yamamoto, T., Miyata, H., Fujiyama, T., Kinoshita, T. & Maki, S. Serum Tamm-Horsfall glycoprotein level in children with various renal diseases. Nephron 59, 440–444 (1991).
Johnstone, L. M., Jones, C. L., Walker, R. G. & Powell, H. R. Tamm-Horsfall protein: are serum levels a marker for urinary tract obstruction? Pediatr. Nephrol. 8, 689–693 (1994).
Delgado, G. E. et al. Serum uromodulin and mortality risk in patients undergoing coronary angiography. J. Am. Soc. Nephrol. 28, 2201–2210 (2017).
Hanson, L. A., Fasth, A. & Jodal, U. Autoantibodies to Tamm-Horsfall protein, a tool for diagnosing the level of urinary-tract infection. Lancet 1, 226–228 (1976).
Marier, R. et al. Antibody to Tamm-Horsfall protein in patients with urinary tract obstruction and vesicoureteral reflux. J. Infect. Dis. 138, 781–790 (1978).
Ooi, B. S. et al. Antibody to Tamm-Horsfall protein after acute tubular necrosis. Am. J. Nephrol. 1, 48–51 (1981).
Fowler, J. E. et al. Serum antibody against Tamm-Horsfall protein in patients with renal cell carcinoma. Cancer 59, 1923–1926 (1987).
Hoyer, J. R. Tubulointerstitial immune complex nephritis in rats immunized with Tamm-Horsfall protein. Kidney Int. 17, 284–292 (1980).
Mayrer, A. R. et al. Tubulointerstitial nephritis and immunologic responses to Tamm-Horsfall protein in rabbits challenged with homologous urine or Tamm-Horsfall protein. J. Immunol. 128, 2634–2642 (1982).
Fasth, A., Hoyer, J. R. & Seiler, M. W. Renal tubular immune complex formation in mice immunized with Tamm-Horsfall protein. Am. J. Pathol. 125, 555–562 (1986).
Fasth, A., Bjure, J., Hellstrom, M., Jacobsson, B. & Jodal, U. Autoantibodies to Tamm-Horsfall glycoprotein in children with renal damage associated with urinary tract infections. Acta Paediatr. Scand. 69, 709–715 (1980).
Lynn, K. L. & Marshall, R. D. The presence in serum of proteins which are immunologically cross-reactive with Tamm-Horsfall glycoprotein. Biochem. J. 194, 561–568 (1981).
Hunt, J. S., Groufsky, A. & Lynn, K. L. Studies to assess the biological relevance of anti-Tamm-Horsfall protein antibodies detected by direct-binding enzyme-linked immunosorbent assay. Clin. Sci. (Lond.) 73, 479–487 (1987).
Pinto, M., Oron, C., Pinto, O. & Peer, G. Natural autoantibodies against Tamm-Horsfall glycoprotein in normal individuals in relation to age and in adult patients with kidney diseases. Jpn. J. Exp. Med. 60, 197–202 (1990).
Kirby, A. et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat. Genet. 45, 299–303 (2013).
Bingham, C. et al. Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1β gene mutation. Kidney Int. 63, 1645–1651 (2003).
Zivna, M. et al. Dominant renin gene mutations associated with early-onset hyperuricemia, anemia, and chronic kidney failure. Am. J. Hum. Genet. 85, 204–213 (2009).
Bolar, N. A. et al. Heterozygous loss-of-function SEC61A1 mutations cause autosomal-dominant tubulo-interstitial and glomerulocystic kidney disease with anemia. Am. J. Hum. Genet. 99, 174–187 (2016).
Piret, S. E. et al. Genome-wide study of familial juvenile hyperuricaemic (gouty) nephropathy (FJHN) indicates a new locus, FJHN3, linked to chromosome 2p22.1-p21. Hum. Genet. 129, 51–58 (2011).
Eckardt, K. U. et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—A KDIGO consensus report. Kidney Int. 88, 676–683 (2015). Consensus report on the diagnosis and classification of autosomal dominant tubulointerstitial kidney disease.
Lhotta, K. et al. Epidemiology of uromodulin-associated kidney disease - results from a nation-wide survey. Nephron Extra 2, 147–158 (2012).
Quaglia, M. et al. Unexpectedly high prevalence of rare genetic disorders in kidney transplant recipients with an unknown causal nephropathy. Clin. Transplant 28, 995–1003 (2014).
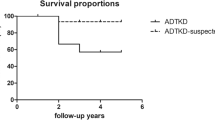
Bollee, G. et al. Phenotype and outcome in hereditary tubulointerstitial nephritis secondary to UMOD mutations. Clin. J. Am. Soc. Nephrol. 6, 2429–2438 (2011). Large clinical series detailing the phenotype of patients harbouring dominant mutations in UMOD.
Moskowitz, J. L. et al. Association between genotype and phenotype in uromodulin-associated kidney disease. Clin. J. Am. Soc. Nephrol. 8, 1349–1357 (2013).
Ekici, A. B. et al. Renal fibrosis is the common feature of autosomal dominant tubulointerstitial kidney diseases caused by mutations in mucin 1 or uromodulin. Kidney Int. 86, 589–599 (2014).
Lens, X. M., Banet, J. F., Outeda, P. & Barrio-Lucia, V. A novel pattern of mutation in uromodulin disorders: autosomal dominant medullary cystic kidney disease type 2, familial juvenile hyperuricemic nephropathy, and autosomal dominant glomerulocystic kidney disease. Am. J. Kidney Dis. 46, 52–57 (2005).
Dahan, K. et al. Familial juvenile hyperuricemic nephropathy and autosomal dominant medullary cystic kidney disease type 2: two facets of the same disease? J. Am. Soc. Nephrol. 12, 2348–2357 (2001).
Vylet'al, P. et al. Alterations of uromodulin biology: a common denominator of the genetically heterogeneous FJHN/MCKD syndrome. Kidney Int. 70, 1155–1169 (2006).
Nasr, S. H., Lucia, J. P., Galgano, S. J., Markowitz, G. S. & D'Agati, V. D. Uromodulin storage disease. Kidney Int. 73, 971–976 (2008).
Bleyer, A. J., Hart, T. C., Shihabi, Z., Robins, V. & Hoyer, J. R. Mutations in the uromodulin gene decrease urinary excretion of Tamm-Horsfall protein. Kidney Int. 66, 974–977 (2004).
Edwards, N. et al. Novel homozygous UMOD mutation reveals gene-dosage effects on uromodulin processing and urinary excretion. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfx066 (2017).
Williams, S. E. et al. Uromodulin mutations causing familial juvenile hyperuricaemic nephropathy lead to protein maturation defects and retention in the endoplasmic reticulum. Hum. Mol. Genet. 18, 2963–2974 (2009).
Bernascone, I. et al. A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure. Hum. Mol. Genet. 19, 2998–3010 (2010). The first transgenic mouse model of uromodulin-associated kidney disease.
Kemter, E. et al. Novel missense mutation of uromodulin in mice causes renal dysfunction with alterations in urea handling, energy, and bone metabolism. Am. J. Physiol. Renal Physiol. 297, F1391–1398 (2009).
Kemter, E. et al. Standardized, systemic phenotypic analysis of Umod(C93F) and Umod(A227T) mutant mice. PLoS ONE 8, e78337 (2013).
Kemter, E., Frohlich, T., Arnold, G. J., Wolf, E. & Wanke, R. Mitochondrial dysregulation secondary to endoplasmic reticulum stress in autosomal dominant tubulointerstitial kidney disease - UMOD (ADTKD-UMOD). Sci. Rep. 7, 42970 (2017).
Piret, S. E. et al. Mouse model for inherited renal fibrosis associated with endoplasmic reticulum stress. Dis. Model. Mech. 10, 773–786 (2017). First knock-in mouse model of uromodulin-associated kidney disease, with ER stress as a key pathogenic mechanism.
Raffi, H., Bates, J. M., Laszik, Z. & Kumar, S. Tamm-Horsfall protein knockout mice do not develop medullary cystic kidney disease. Kidney Int. 69, 1914–1915 (2006).
Rezende-Lima, W. et al. Homozygosity for uromodulin disorders: FJHN and MCKD-type 2. Kidney Int. 66, 558–563 (2004).
Choi, S. W. et al. Mutant tamm-horsfall glycoprotein accumulation in endoplasmic reticulum induces apoptosis reversed by colchicine and sodium 4-phenylbutyrate. J. Am. Soc. Nephrol. 16, 3006–3014 (2005).
Utami, S. B. et al. Apoptosis induced by an uromodulin mutant C112Y and its suppression by topiroxostat. Clin. Exp. Nephrol. 19, 576–584 (2015).
Kemter, E. et al. No amelioration of uromodulin maturation and trafficking defect by sodium 4-phenylbutyrate in vivo: studies in mouse models of uromodulin-associated kidney disease. J. Biol. Chem. 289, 10715–10726 (2014).
Glaudemans, B. et al. A primary culture system of mouse thick ascending limb cells with preserved function and uromodulin processing. Pflugers Arch. 466, 343–356 (2014). First primary culture system of TAL cells, which enabled studies of endogenous uromodulin properties.
Kottgen, A. et al. New loci associated with kidney function and chronic kidney disease. Nat. Genet. 42, 376–384 (2010).
Chambers, J. C. et al. Genetic loci influencing kidney function and chronic kidney disease. Nat. Genet. 42, 373–375 (2010).
Pattaro, C. et al. Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet. 8, e1002584 (2012).
Sveinbjornsson, G. et al. Rare mutations associating with serum creatinine and chronic kidney disease. Hum. Mol. Genet. 23, 6935–6943 (2014).
Liu, C. T. et al. Genetic association for renal traits among participants of African ancestry reveals new loci for renal function. PLoS Genet. 7, e1002264 (2011).
Okada, Y. et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat. Genet. 44, 904–909 (2012).
Boger, C. A. et al. Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD. PLoS Genet. 7, e1002292 (2011).
Gorski, M. et al. Genome-wide association study of kidney function decline in individuals of European descent. Kidney Int. 87, 1017–1029 (2015).
Ahluwalia, T. S., Lindholm, E., Groop, L. & Melander, O. Uromodulin gene variant is associated with type 2 diabetic nephropathy. J. Hypertens. 29, 1731–1734 (2011).
Deshmukh, H. A., Palmer, C. N., Morris, A. D. & Colhoun, H. M. Investigation of known estimated glomerular filtration rate loci in patients with type 2 diabetes. Diabet. Med. 30, 1230–1235 (2013).
Guan, M. et al. Association of kidney structure-related gene variants with type 2 diabetes-attributed end-stage kidney disease in African Americans. Hum. Genet. 135, 1251–1262 (2016).
Shlipak, M. G. & Day, E. C. Biomarkers for incident CKD: a new framework for interpreting the literature. Nat. Rev. Nephrol. 9, 478–483 (2013).
Han, J. et al. Common genetic variants of the human uromodulin gene regulate transcription and predict plasma uric acid levels. Kidney Int. 83, 733–740 (2013).
Wuttke, M. & Kottgen, A. Insights into kidney diseases from genome-wide association studies. Nat. Rev. Nephrol. 12, 549–562 (2016).
Eckardt, K. U. et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 382, 158–169 (2013).
Kottgen, A. et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J. Am. Soc. Nephrol. 21, 337–344 (2010).
Devuyst, O. Salt wasting and blood pressure. Nat. Genet. 40, 495–496 (2008).
Di Rienzo, A. & Hudson, R. R. An evolutionary framework for common diseases: the ancestral-susceptibility model. Trends Genet. 21, 596–601 (2005).
Rossier, B. C., Bochud, M. & Devuyst, O. The hypertension pandemic: an evolutionary perspective. Physiol. (Bethesda) 32, 112–125 (2017). Global and evolutionary perspectives about the role of uromodulin in salt handling.
Genovese, G. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010).
Raffi, H. S., Bates, J. M. Jr., Laszik, Z. & Kumar, S. Tamm-horsfall protein protects against urinary tract infection by proteus mirabilis. J. Urol. 181, 2332–2338 (2009).
Mo, L. et al. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. Am. J. Physiol. Renal Physiol. 293, F1935–F1943 (2007).
Liu, Y., El-Achkar, T. M. & Wu, X. R. Tamm-Horsfall protein regulates circulating and renal cytokines by affecting glomerular filtration rate and acting as a urinary cytokine trap. J. Biol. Chem. 287, 16365–16378 (2012).
Kemter, E. et al. Type of uromodulin mutation and allelic status influence onset and severity of uromodulin-associated kidney disease in mice. Hum. Mol. Genet. 22, 4148–4163 (2013).
Mahajan, A. et al. Trans-ethnic fine mapping highlights kidney-function genes linked to salt sensitivity. Am. J. Hum. Genet. 99, 636–646 (2016).
Acknowledgements
O.D. is supported by grants from the European Community's Seventh Framework Programme (305608 EURenOmics), the Swiss National Centre of Competence in Research Kidney Control of Homeostasis (NCCR Kidney.CH) programme, the Swiss National Science Foundation (31003A_169850) and the Rare Disease Initiative Zürich (Radiz), a clinical research priority programme of the University of Zürich, Switzerland. E.O. is supported by the Fonds National de la Recherche Luxembourg (6903109), and the University Research Priority Programme “Integrative Human Physiology, ZIHP” of the University of Zürich. L.R. is supported by grants from Telethon-Italy (TCR08006, GGP14263), the Italian Ministry of Health (RF-2010-2319394) and Fondazione Cariplo (2014–0827). We are grateful to Gregor Weiss (ETH, Zurich) for providing EM pictures of uromodulin, to Céline Schaeffer (San Raffaele, Milan) for reviewing the UMOD mutations and to Sonia Youhanna (UZH, Zurich) for helpful assistance in deglycosylation experiments.
Author information
Authors and Affiliations
Contributions
O.D., E.O. and L.R. researched the data, discussed the article content and wrote, edited and approved the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Devuyst, O., Olinger, E. & Rampoldi, L. Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol 13, 525–544 (2017). https://doi.org/10.1038/nrneph.2017.101
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.101
This article is cited by
-
Pregnancy-associated changes in urinary uromodulin excretion in chronic hypertension
Journal of Nephrology (2024)
-
Hidden genetics behind glomerular scars: an opportunity to understand the heterogeneity of focal segmental glomerulosclerosis?
Pediatric Nephrology (2024)
-
Cardiovascular risk and kidney function profiling using conventional and novel biomarkers in young adults: the African-PREDICT study
BMC Nephrology (2023)
-
Association of clinical characteristics with urine uromodulin in children with chronic kidney disease
Pediatric Nephrology (2023)
-
Knowledge mapping of UMOD of English published work from 1985 to 2022: a bibliometric analysis
International Urology and Nephrology (2023)