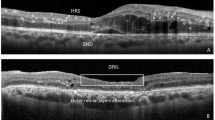
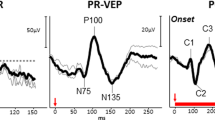
Key Points
-
Inherited neurodegenerative diseases are associated with a wide range of ocular abnormalities, which can cause substantial disability
-
Ophthalmic findings can aid the genetic diagnosis of some neurodegenerative conditions; this diagnosis might, in turn, direct the clinician towards careful examination of the visual systems to provide useful adjunctive information
-
In some patients, ophthalmic manifestations are the earliest symptoms of inherited neurodegenerative disease
-
An increasing body of evidence indicates that ophthalmic findings can act as surrogate markers of disease progression in patients with inherited neurodegenerative conditions
-
Optical coherence tomography is emerging as a useful tool for quantifying retinal and optic nerve findings in patients with neurodegenerative disease
-
The role of mitochondrial dysfunction in inherited neurodegenerative disease has been well established, and ophthalmic involvement is a common manifestation of this dysfunction
Abstract
Ophthalmic findings are common features of neurodegenerative disorders and, in addition to being clinically important, have emerged as potentially useful biomarkers of disease progression in several conditions. Clinically, these visual system abnormalities can be a clue to diagnosis, as well as being a prominent cause of disability in affected patients. In this Review, we describe the various afferent visual system and other ophthalmic features of inherited neurodegenerative disorders, including the muscular dystrophies, Friedreich ataxia, the spinocerebellar ataxias, hereditary spastic paraplegia, Charcot–Marie–Tooth disease, and other conditions. We focus on the expanding role of optical coherence tomography in diagnostic imaging of the retina and optic nerve head, and the possible use of ophthalmic findings as biomarkers of disease severity in hereditary neurodegenerative disorders. In addition, we discuss the ophthalmic manifestations and treatment implications of mitochondrial dysfunction, which is a feature of many inherited neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chung, K. W. et al. Early onset severe and late-onset mild Charcot–Marie–Tooth disease with mitofusin 2 (MFN2) mutations. Brain 129, 2103–2118 (2006).
Fortuna, F. et al. Visual system involvement in patients with Friedreich's ataxia. Brain 132, 116–123 (2009).
Klebe, S. et al. Spastic paraplegia gene 7 in patients with spasticity and/or optic neuropathy. Brain 135, 2980–2993 (2012).
Pula, J. H., Gomez, C. M. & Kattah, J. C. Ophthalmologic features of the common spinocerebellar ataxias. Curr. Opin. Ophthalmol. 21, 447–453 (2010).
Grainger, B. T., Papchenko, T. L. & Danesh-Meyer, H. V. Optic nerve atrophy in adrenoleukodystrophy detectable by optic coherence tomography. J. Clin. Neurosci. 17, 122–124 (2010).
Diago, T., Valls, B. & Pulido, J. S. Coats' disease associated with muscular dystrophy treated with ranibizumab. Eye (Lond.) 24, 1295–1296 (2010).
Puech, B. et al. Kjellin syndrome: long-term neuro-ophthalmologic follow-up and novel mutations in the SPG11 gene. Ophthalmology 118, 564–573 (2011).
Fitzsimons, R. B., Gurwin, E. B. & Bird, A. C. Retinal vascular abnormalities in facioscapulohumeral muscular dystrophy. A general association with genetic and therapeutic implications. Brain 110, 631–648 (1987).
Aleman, T. S. et al. Spinocerebellar ataxia type 7 (SCA7) shows a cone–rod dystrophy phenotype. Exp. Eye Res. 74, 737–745 (2002).
Kersten, H. M. et al. Epiretinal membrane: a treatable cause of visual disability in myotonic dystrophy type 1. J. Neurol. 261, 37–44 (2014).
Yu-Wai-Man, P., Griffiths, P. G. & Chinnery, P. F. Mitochondrial optic neuropathies—disease mechanisms and therapeutic strategies. Prog. Retin. Eye Res. 30, 81–114 (2011).
Frohman, E. M. et al. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat. Clin. Pract. Neurol. 4, 664–675 (2008).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Greenfield, D. S. & Weinreb, R. N. Role of optic nerve imaging in glaucoma clinical practice and clinical trials. Am. J. Ophthalmol. 145, 598–603 (2008).
Milani, P., Raimondi, G., Morale, D. & Scialdone, A. Biomicroscopy versus optical coherence tomography screening of epiretinal membranes in patients undergoing cataract surgery. Retina 32, 897–904 (2012).
Lalwani, G. A. et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am. J. Ophthalmol. 148, 43–58.e1 (2009).
Marziani, E. et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer's disease using spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 54, 5953–5958 (2013).
Satue, M. et al. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson's disease patients. Eye (Lond.) 27, 507–514 (2013).
Anderson, T. J. & MacAskill, M. R. Eye movements in patients with neurodegenerative disorders. Nat. Rev. Neurol. 9, 74–85 (2013).
Emery, A. E. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul. Disord. 1, 19–29 (1991).
Brook, J. D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 69, 385 (1992).
Buxton, J. et al. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature 355, 547–548 (1992).
Groh, W. J., Lowe, M. R. & Zipes, D. P. Severity of cardiac conduction involvement and arrhythmias in myotonic dystrophy type 1 correlates with age and CTG repeat length. J. Cardiovasc. Electrophysiol. 13, 444–448 (2002).
Harley, H. G. et al. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am. J. Hum. Genet. 52, 1164–1174 (1993).
Rosa, N. et al. Low intraocular pressure resulting from ciliary body detachment in patients with myotonic dystrophy. Ophthalmology 118, 260–264 (2011).
Betten, M. G., Bilchik, R. C. & Smith, M. E. Pigmentary retinopathy of myotonic dystrophy. Am. J. Ophthalmol. 72, 720–723 (1971).
Eshaghian, J., March, W. F., Goossens, W. & Rafferty, N. S. Ultrastructure of cataract in myotonic dystrophy. Invest. Ophthalmol. Vis. Sci. 17, 289–293 (1978).
Doherty, M., Winterton, R. & Griffiths, P. G. Eyelid surgery in ocular myopathies. Orbit 32, 12–15 (2013).
Liquori, C. L. et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 293, 864–867 (2001).
Ranum, L. P. & Day, J. W. Myotonic dystrophy: clinical and molecular parallels between myotonic dystrophy type 1 and type 2. Curr. Neurol. Neurosci. Rep. 2, 465–470 (2002).
Ginsberg, J., Hamblet, J. & Menefee, M. Ocular abnormality in myotonic dystrophy. Ann. Ophthalmol. 10, 1021–1028 (1978).
Yanoff, M. & Fine, B. S. Ocular Pathology: A Text and Atlas 2nd edn (Harper & Row, 1982).
Sarks, J. et al. Retinal changes in myotonic dystrophy: a clinicomorphological study. Aust. N. Z. J. Ophthalmol. 13, 19–36 (1985).
Burian, H. M. & Burns, C. A. Ocular changes in myotonic dystrophy. Am. J. Ophthalmol. 63, 22–34 (1967).
Ghazi-Nouri, S. M., Tranos, P. G., Rubin, G. S., Adams, Z. C. & Charteris, D. G. Visual function and quality of life following vitrectomy and epiretinal membrane peel surgery. Br. J. Ophthalmol. 90, 559–562 (2006).
Gamez, J., Montane, D., Martorell, L., Minoves, T. & Cervera, C. Bilateral optic nerve atrophy in myotonic dystrophy. Am. J. Ophthalmol. 131, 398–400 (2001).
Ashizawa, T. et al. Diagnostic value of ophthalmologic findings in myotonic dystrophy: comparison with risks calculated by haplotype analysis of closely linked restriction fragment length polymorphisms. Am. J. Med. Genet. 42, 55–60 (1992).
Schara, U. & Schoser, B. G. Myotonic dystrophies type 1 and 2: a summary on current aspects. Semin. Pediatr. Neurol. 13, 71–79 (2006).
Vos, T. A. 25 years dystrophia myotonica (D.M.). Ophthalmologica 141, 37–45 (1961).
Hayasaka, S. et al. Ciliary and retinal changes in myotonic dystrophy. Arch. Ophthalmol. 102, 88–93 (1984).
Khan, A. R. & Brubaker, R. F. Aqueous humor flow and flare in patients with myotonic dystrophy. Invest. Ophthalmol. Vis. Sci. 34, 3131–3139 (1993).
Walker, S. D., Brubaker, R. F. & Nagataki, S. Hypotony and aqueous humor dynamics in myotonic dystrophy. Invest. Ophthalmol. Vis. Sci. 22, 744–751 (1982).
Rosa, N. et al. Corneal thickness and endothelial cell characteristics in patients with myotonic dystrophy. Ophthalmology 117, 223–225 (2010).
Wong, V. A., Beckingsale, P. S., Oley, C. A. & Sullivan, T. J. Management of myogenic ptosis. Ophthalmology 109, 1023–1031 (2002).
Bollinger, K. E. et al. Hypermetropia and esotropia in myotonic dystrophy. J. AAPOS 12, 69–71 (2008).
Ekstrom, A.-B., Tulinius, M., Sjostrom, A. & Aring, E. Visual function in congenital and childhood myotonic dystrophy type 1. Ophthalmology 117, 976–982 (2010).
Tawil, R. Facioscapulohumeral muscular dystrophy. Neurotherapeutics 5, 601–606 (2008).
Tawil, R., Figlewicz, D. A., Griggs, R. C. & Weiffenbach, B. Facioscapulohumeral dystrophy: a distinct regional myopathy with a novel molecular pathogenesis. FSH Consortium. Ann. Neurol. 43, 279–282 (1998).
Wijmenga, C. et al. Location of facioscapulohumeral muscular dystrophy gene on chromosome 4. Lancet 336, 651–653 (1990).
van Deutekom, J. C. et al. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum. Mol. Genet. 2, 2037–2042 (1993).
Bengtsson, U., Altherr, M. R., Wasmuth, J. J. & Winokur, S. T. High resolution fluorescence in situ hybridization to linearly extended DNA visually maps a tandem repeat associated with facioscapulohumeral muscular dystrophy immediately adjacent to the telomere of 4q. Hum. Mol. Genet. 3, 1801–1805 (1994).
Gurwin, E. B., Fitzsimons, R. B., Sehmi, K. S. & Bird, A. C. Retinal telangiectasis in facioscapulohumeral muscular dystrophy with deafness. Arch. Ophthalmol. 103, 1695–1700 (1985).
Taylor, D. A. et al. Facioscapulohumeral dystrophy associated with hearing loss and Coats syndrome. Ann. Neurol. 12, 395–398 (1982).
Bindoff, L. A. et al. Severe fascioscapulohumeral muscular dystrophy presenting with Coats' disease and mental retardation. Neuromuscul. Disord. 16, 559–563 (2006).
Shields, C. L. et al. Neovascular glaucoma from advanced Coats disease as the initial manifestation of facioscapulohumeral dystrophy in a 2-year-old child. Arch. Ophthalmol. 125, 840–842 (2007).
Fitzsimons, R. B. Retinal vascular disease and the pathogenesis of facioscapulohumeral muscular dystrophy. A signalling message from Wnt? Neuromuscul. Disord. 21, 263–271 (2011).
Rosa, N. et al. Intraocular pressure in patients with muscular dystrophies. Ophthalmology 120, 1306–1307.e1 (2013).
Shy, M. E., Garbern, J. Y. & Kamholz, J. Hereditary motor and sensory neuropathies: a biological perspective. Lancet Neurol. 1, 110–118 (2002).
Zuchner, S. & Vance, J. M. Mechanisms of disease: a molecular genetic update on hereditary axonal neuropathies. Nat. Clin. Pract. Neurol. 2, 45–53 (2006).
Pareyson, D. & Marchesi, C. Diagnosis, natural history, and management of Charcot–Marie–Tooth disease. Lancet Neurol. 8, 654–667 (2009).
Timmerman, V., Clowes, V. E. & Reid, E. Overlapping molecular pathological themes link Charcot–Marie–Tooth neuropathies and hereditary spastic paraplegias. Exp. Neurol. 246, 14–25 (2013).
Li, J. Inherited neuropathies. Semin. Neurol. 32, 204–214 (2012).
Zuchner, S. et al. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann. Neurol. 59, 276–281 (2006).
Zuchner, S. et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot–Marie–Tooth neuropathy type 2A. Nat. Genet. 36, 449–451 (2004).
Pich, S. et al. The Charcot–Marie–Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum. Mol. Genet. 14, 1405–1415 (2005).
Chen, H. & Chan, D. C. Critical dependence of neurons on mitochondrial dynamics. Curr. Opin. Cell Biol. 18, 453–459 (2006).
Loiseau, D. et al. Mitochondrial coupling defect in Charcot–Marie–Tooth type 2A disease. Ann. Neurol. 61, 315–323 (2007).
Brockmann, K. et al. Cerebral involvement in axonal Charcot–Marie–Tooth neuropathy caused by mitofusin2 mutations. J. Neurol. 255, 1049–1058 (2008).
Houlden, H., Reilly, M. M. & Smith, S. Pupil abnormalities in 131 cases of genetically defined inherited peripheral neuropathy. Eye (Lond.) 23, 966–974 (2009).
Yu-Wai-Man, P. et al. Multi-system neurological disease is common in patients with OPA1 mutations. Brain 133, 771–786 (2010).
Gowrisankaran, S., Anastasakis, A., Fishman, G. A. & Alexander, K. R. Structural and functional measures of inner retinal integrity following visual acuity improvement in a patient with hereditary motor and sensory neuropathy type VI. Ophthalmic Genet. 32, 188–192 (2011).
Campuzano, V. et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427 (1996).
Harding, A. E. Clinical features and classification of inherited ataxias. Adv. Neurol. 61, 1–14 (1993).
Durr, A. et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N. Engl. J. Med. 335, 1169–1175 (1996).
Dunaief, J. L. Ironing out neurodegeneration: iron chelation for neuroprotection. Free Radic. Biol. Med. 51, 1480–1481 (2011).
Fahey, M. C. et al. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain 131, 1035–1045 (2008).
Furman, J. M., Perlman, S. & Baloh, R. W. Eye movements in Friedreich's ataxia. Arch. Neurol. 40, 343–346 (1983).
Hocking, D. R. et al. Ocular motor fixation deficits in Friedreich ataxia. Cerebellum 9, 411–418 (2010).
Spieker, S. et al. Fixation instability and oculomotor abnormalities in Friedreich's ataxia. J. Neurol. 242, 517–521 (1995).
Carroll, W. M., Kriss, A., Baraitser, M., Barrett, G. & Halliday, A. M. The incidence and nature of visual pathway involvement in Friedreich's ataxia. A clinical and visual evoked potential study of 22 patients. Brain 103, 413–434 (1980).
Noval, S., Contreras, I., Sanz-Gallego, I., Manrique, R. K. & Arpa, J. Ophthalmic features of Friedreich ataxia. Eye (Lond.) 26, 315–320 (2012).
Seyer, L. A. et al. Analysis of the visual system in Friedreich ataxia. J. Neurol. 260, 2362–2369 (2013).
Duenas, A. M., Goold, R. & Giunti, P. Molecular pathogenesis of spinocerebellar ataxias. Brain 129, 1357–1370 (2006).
Paulson, H. L. The spinocerebellar ataxias. J. Neuroophthalmol. 29, 227–237 (2009).
Lynch, D. R. & Farmer, J. Practical approaches to neurogenetic disease. J. Neuroophthalmol. 22, 297–304 (2002).
Moschner, C., Perlman, S. & Baloh, R. W. Comparison of oculomotor findings in the progressive ataxia syndromes. Brain 117, 15–25 (1994).
Manto, M.-U. The wide spectrum of spinocerebellar ataxias (SCAs). Cerebellum 4, 2–6 (2005).
Vaclavik, V., Borruat, F.-X., Ambresin, A. & Munier, F. L. Novel maculopathy in patients with spinocerebellar ataxia type 1 autofluorescence findings and functional characteristics. JAMA Ophthalmol. 131, 536–538 (2013).
Abe, T., Abe, K., Aoki, M., Itoyama, Y. & Tamai, M. Ocular changes in patients with spinocerebellar degeneration and repeated trinucleotide expansion of spinocerebellar ataxia type 1 gene. Arch. Ophthalmol. 115, 231–236 (1997).
Lee, W. Y. et al. Frequency analysis and clinical characterization of spinocerebellar ataxia types 1, 2, 3, 6, and 7 in Korean patients. Arch. Neurol. 60, 858–863 (2003).
Stricker, S. et al. Temporal retinal nerve fiber loss in patients with spinocerebellar ataxia type 1. PLoS ONE 6 (2011).
Pogacar, S., Ambler, M., Conklin, W. J., O'Neil, W. A. & Lee, H. Y. Dominant spinopontine atrophy. Report of two additional members of family W. Arch. Neurol. 35, 156–162 (1978).
Alvarez, G. et al. Optical coherence tomography findings in spinocerebellar ataxia-3. Eye (Lond.) 27, 1376–1381 (2013).
Jardim, L. B. et al. Neurologic findings in Machado–Joseph disease: relation with disease duration, subtypes, and (CAG)n . Arch. Neurol. 58, 899–904 (2001).
Pula, J. H. et al. Retinal nerve fibre layer and macular thinning in spinocerebellar ataxia and cerebellar multisystem atrophy. Neuroophthalmology 35, 108–114 (2011).
Miller, R. C., Tewari, A., Miller, J. A., Garbern, J. & Van Stavern, G. P. Neuro-ophthalmologic features of spinocerebellar ataxia type 7. J. Neuroophthalmology 29, 180–186 (2009).
Michalik, A., Martin, J. J. & Van Broeckhoven, C. Spinocerebellar ataxia type 7 associated with pigmentary retinal dystrophy. Eur. J. Hum. Genet. 12, 2–15 (2004).
Thurtell, M. J. et al. Two patients with spinocerebellar ataxia type 7 presenting with profound binocular visual loss yet minimal ophthalmoscopic findings. J. Neuroophthalmol. 29, 187–191 (2009).
Ahn, J. K., Seo, J.-M., Chung, H. & Yu, H. G. Anatomical and functional characteristics in atrophic maculopathy associated with spinocerebellar ataxia type 7. Am. J. Ophthalmol. 139, 923–925 (2005).
Hugosson, T., Granse, L., Ponjavic, V. & Andreasson, S. Macular dysfunction and morphology in spinocerebellar ataxia type 7 (SCA 7). Ophthalmic Genet. 30, 1–6 (2009).
Horton, L. C. et al. Spinocerebellar ataxia type 7: clinical course, phenotype–genotype correlations, and neuropathology. Cerebellum 12, 176–193 (2013).
Bouchard, J. P. et al. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Neuromuscul. Disord. 8, 474–479 (1998).
Gerwig, M. et al. Characteristic MRI and funduscopic findings help diagnose ARSACS outside Quebec. Neurology 75, 2133 (2010).
Takiyama, Y. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Neuropathology 26, 368–375 (2006).
Garcia-Martin, E. et al. Retinal segmentation as noninvasive technique to demonstrate hyperplasia in ataxia of Charlevoix-Saguenay. Invest. Ophthalmol. Vis. Sci. 54, 7137–7142 (2013).
Baets, J. et al. Mutations in SACS cause atypical and late-onset forms of ARSACS. Neurology 75, 1181–1188 (2010).
Ogawa, T. et al. Identification of a SACS gene missense mutation in ARSACS. Neurology 62, 107–109 (2004).
Garcia-Martin, E. et al. Retinal nerve fibre layer thickness in ARSACS: myelination or hypertrophy? Br. J. Ophthalmol. 97, 238–241 (2013).
Vingolo, E. M. et al. Myelinated retinal fibers in autosomal recessive spastic ataxia of Charlevoix-Saguenay. Eur. J. Neurol. 18, 1187–1190 (2011).
Desserre, J. et al. Thickening of peripapillar retinal fibers for the diagnosis of autosomal recessive spastic ataxia of Charlevoix-Saguenay. Cerebellum 10, 758–762 (2011).
Girard, M. et al. Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS). Proc. Natl Acad. Sci. USA 109, 1661–1666 (2012).
Harding, A. E. Classification of the hereditary ataxias and paraplegias. Lancet 1, 1151–1155 (1983).
Salinas, S., Proukakis, C., Crosby, A. & Warner, T. T. Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol. 7, 1127–1138 (2008).
Schule, R. & Schols, L. Genetics of hereditary spastic paraplegias. Semin. Neurol. 31, 484–493 (2011).
Fink, J. K. Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms. Acta Neuropathol. 126, 307–328 (2013).
Roxburgh, R. H. et al. The p.Ala510Val mutation in the SPG7 (paraplegin) gene is the most common mutation causing adult onset neurogenetic disease in patients of British ancestry. J. Neurol. 260, 1286–1294 (2013).
Wiethoff, S., Zhour, A., Schöls, L. & Fischer, M. D. Retinal nerve fibre layer loss in hereditary spastic paraplegias is restricted to complex phenotypes. BMC Neurol. 12, 143 (2012).
Casari, G. et al. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell 93, 973–983 (1998).
Martinelli, P. & Rugarli, E. I. Emerging roles of mitochondrial proteases in neurodegeneration. Biochim. Biophys. Acta 1797, 1–10 (2010).
Pellegrini, L. & Scorrano, L. A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death Differ. 14, 1275–1284 (2007).
Shimazaki, H. et al. A homozygous mutation of C12orf65 causes spastic paraplegia with optic atrophy and neuropathy (SPG55). J. Med. Genet. 49, 777–784 (2012).
Stevanin, G. et al. Mutations in SPG11, encoding spatacsin, are a major cause of spastic paraplegia with thin corpus callosum. Nat. Genet. 39, 366–372 (2007).
Hanein, S. et al. Identification of the SPG15 gene, encoding spastizin, as a frequent cause of complicated autosomal-recessive spastic paraplegia, including Kjellin syndrome. Am. J. Hum. Genet. 82, 992–1002 (2008).
Webb, S., Patterson, V. & Hutchinson, M. Two families with autosomal recessive spastic paraplegia, pigmented maculopathy, and dementia. J. Neurol. Neurosurg. Psychiatry 63, 628–632 (1997).
Dusek, P. & Schneider, S. A. Neurodegeneration with brain iron accumulation. Curr. Opin. Neurol. 25, 499–506 (2012).
Hartig, M. B. et al. Absence of an orphan mitochondrial protein, c19orf12, causes a distinct clinical subtype of neurodegeneration with brain iron accumulation. Am. J. Hum. Genet. 89, 543–550 (2011).
Dezfouli, M. A. et al. PANK2 and C19orf12 mutations are common causes of neurodegeneration with brain iron accumulation. Mov. Disord. 28, 228–232 (2013).
Kazek, B. et al. A novel PANK2 gene mutation: clinical and molecular characteristics of patients short communication. J. Child Neurol. 22, 1256–1259 (2007).
Hartig, M. B., Prokisch, H., Meitinger, T. & Klopstock, T. Pantothenate kinase-associated neurodegeneration. Curr. Drug Targets 13, 1182–1189 (2012).
Zhou, B. et al. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden–Spatz syndrome. Nat. Genet. 28, 345–349 (2001).
Mitamura, Y. et al. Diagnostic imaging in patients with retinitis pigmentosa. J. Med. Invest. 59, 1–11 (2012).
Hartig, M., Prokisch, H., Meitinger, T. & Klopstock, T. Mitochondrial membrane protein-associated neurodegeneration (MPAN). Int. Rev. Neurobiol. 110, 73–84 (2013).
Horvath, R. et al. A new phenotype of brain iron accumulation with dystonia, optic atrophy, and peripheral neuropathy. Mov. Disord. 27, 789–793 (2012).
Kruer, M. C. et al. C19orf12 mutation leads to a pallido-pyramidal syndrome. Gene 537, 352–356 (2014).
Schulte, E. C. et al. Mitochondrial membrane protein associated neurodegenration: a novel variant of neurodegeneration with brain iron accumulation. Mov. Disord. 28, 224–227 (2013).
Wolkow, N. et al. Aceruloplasminemia: retinal histopathologic manifestations and iron-mediated melanosome degradation. Arch. Ophthalmol. 129, 1466–1474 (2011).
Moser, H. W. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain 120, 1485–1508 (1997).
Semmler, A., Kohler, W., Jung, H. H., Weller, M. & Linnebank, M. Therapy of X-linked adrenoleukodystrophy. Expert Rev. Neurother. 8, 1367–1379 (2008).
Mosser, J. et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 (1993).
Schaumburg, H. H., Powers, J. M., Raine, C. S., Suzuki, K. & Richardson, E. P. Jr. Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch. Neurol. 32, 577–591 (1975).
Kaplan, P. W. et al. Visual system abnormalities in adrenomyeloneuropathy. Ann. Neurol. 37, 550–552 (1995).
Traboulsi, E. I. & Maumenee, I. H. Ophthalmologic manifestations of X-linked childhood adrenoleukodystrophy. Ophthalmology 94, 47–52 (1987).
Sack, G. H. Jr, Raven, M. B. & Moser, H. W. Color vision defects in adrenomyeloneuropathy. Am. J. Hum. Genet. 44, 794–798 (1989).
Wray, S. H., Cogan, D. G., Kuwabara, T., Schaumburg, H. H. & Powers, J. M. Adrenoleukodystrophy with disease of the eye and optic nerve. Am. J. Ophthalmol. 82, 480–485 (1976).
Wilson, W. B. The visual system manifestations of adrenoleukodystrophy. Neuroophthalmology 1, 175–183 (1981).
Cohen, S. M. et al. Ocular histopathologic studies of neonatal and childhood adrenoleukodystrophy. Am. J. Ophthalmol. 95, 82–96 (1983).
Glasgow, B. J., Brown, H. H., Hannah, J. B. & Foos, R. Y. Ocular pathologic findings in neonatal adrenoleukodystrophy. Ophthalmology 94, 1054–1060 (1987).
Goedert, M. & Spillantini, M. G. A century of Alzheimer's disease. Science 314, 777–781 (2006).
Alonso Vilatela, M. E., Lopez-Lopez, M. & Yescas-Gomez, P. Genetics of Alzheimer's disease. Arch. Med. Res. 43, 622–631 (2012).
Calkins, M. J., Manczak, M., Mao, P., Shirendeb, U. & Reddy, P. H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum. Mol. Genet. 20, 4515–4529 (2011).
Bekris, L. M., Yu, C.-E., Bird, T. D. & Tsuang, D. W. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 23, 213–227 (2010).
Campion, D. et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 65, 664–670 (1999).
Lu, Y. et al. Retinal nerve fiber layer structure abnormalities in early Alzheimer's disease: evidence in optical coherence tomography. Neurosci. Lett. 480, 69–72 (2010).
Danesh-Meyer, H. V., Birch, H. F., Ku, J. Y., Carroll, S. M. & Gamble, G. M. Reduction of optic nerve fibers in patients with Alzheimer disease identified by laser imaging. Neurology 67, 1852–1854 (2006).
Tsai, C. S. et al. Optic nerve head and nerve fiber layer in Alzheimer's disease. Arch. Ophthalmol. 109, 199–204 (1991).
Iseri, P. K., Altinas, O., Tokay, T. & Yuksel, N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J. Neuroophthalmol. 26, 18–24 (2006).
Berisha, F., Feke, G. T., Trempe, C. L., McMeel, J. W. & Schepens, C. L. Retinal abnormalities in early Alzheimer's disease. Invest. Ophthalmol. Vis. Sci. 48, 2285–2289 (2007).
Paquet, C. et al. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Neurosci. Lett. 420, 97–99 (2007).
Valenti, D. A. Neuroimaging of retinal nerve fiber layer in AD using optical coherence tomography. Neurology 69, 1060 (2007).
Kesler, A., Vakhapova, V., Korczyn, A. D., Naftaliev, E. & Neudorfer, M. Retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Clin. Neurol. Neurosurg. 113, 523–526 (2011).
Kirbas, S., Turkyilmaz, K., Anlar, O., Tufekci, A. & Durmus, M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J. Neuroophthalmol. 33, 58–61 (2013).
Mizuno, Y. et al. Progress in the pathogenesis and genetics of Parkinson's disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2215–2227 (2008).
Singleton, A. B., Farrer, M. J. & Bonifati, V. The genetics of Parkinson's disease: progress and therapeutic implications. Mov. Disord. 28, 14–23 (2013).
Fujioka, S. & Wszolek, Z. K. Update on genetics of parkinsonism. Neurodegener. Dis. 10, 257–260 (2012).
Altintas, O., Iseri, P., Ozkan, B. & Caglar, Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson's disease. Doc. Ophthalmol. 116, 137–146 (2008).
Cubo, E., Tedejo, R. P., Rodriguez Mendez, V., Lopez Pena, M. J. & Trejo Gabriel Y Galan, J. M. Retina thickness in Parkinson's disease and essential tremor. Mov. Disord. 25, 2461–2462 (2010).
La Morgia, C. et al. Loss of temporal retinal nerve fibers in Parkinson disease: a mitochondrial pattern? Eur. J. Neurol. 20, 198–201 (2013).
Inzelberg, R., Ramirez, J. A., Nisipeanu, P. & Ophir, A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res. 44, 2793–2797 (2004).
Garcia-Martin, E. et al. Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson's disease. Ophthalmology 119, 2161–2167 (2012).
Satue, M. et al. Retinal thinning and correlation with functional disability in patients with Parkinson's disease. Br. J. Ophthalmol. 98, 350–355 (2013).
Albrecht, P. et al. Optical coherence tomography in parkinsonian syndromes. PLoS ONE 7, e34891 (2012).
Tsironi, E. E. et al. Perimetric and retinal nerve fiber layer findings in patients with Parkinson's disease. BMC Ophthalmol. 12, 54 (2012).
Archibald, N. K., Clarke, M. P., Mosimann, U. P. & Burn, D. J. Retinal thickness in Parkinson's disease. Parkinsonism Relat. Disord. 17, 431–436 (2011).
Archibald, N. K., Clarke, M. P., Mosimann, U. P. & Burn, D. J. Visual symptoms in Parkinson's disease and Parkinson's disease dementia. Mov. Disord. 26, 2387–2395 (2011).
Adam, C. R., Shrier, E., Ding, Y., Glazman, S. & Bodis-Wollner, I. Correlation of inner retinal thickness evaluated by spectral-domain optical coherence tomography and contrast sensitivity in Parkinson disease. J. Neuroophthalmol. 33, 137–142 (2013).
Uc, E. Y. et al. Visual dysfunction in Parkinson disease without dementia. Neurology 65, 1907–1913 (2005).
Karson, C. N., LeWitt, P. A., Calne, D. B. & Wyatt, R. J. Blink rates in Parkinsonism. Ann. Neurol. 12, 580–583 (1982).
Agostino, R. et al. Voluntary, spontaneous, and reflex blinking in Parkinson's disease. Mov. Disord. 23, 669–675 (2008).
Reddy, V. C., Patel, S. V., Hodge, D. O. & Leavitt, J. A. Corneal sensitivity, blink rate, and corneal nerve density in progressive supranuclear palsy and Parkinson disease. Cornea 32, 631–635 (2013).
Sadun, A. A., La Morgia, C. & Carelli, V. Mitochondrial optic neuropathies: our travels from bench to bedside and back again. Clin. Experiment. Ophthalmol. 41, 702–712 (2013).
Debrosse, S. & Parikh, S. Neurologic disorders due to mitochondrial DNA mutations. Semin. Pediatr. Neurol. 19, 194–202 (2012).
Wallace, D. C. Mitochondrial diseases in man and mouse. Science 283, 1482–1488 (1999).
Sitarz, K. S., Chinnery, P. F. & Yu-Wai-Man, P. Disorders of the optic nerve in mitochondrial cytopathies: new ideas on pathogenesis and therapeutic targets. Curr. Neurol. Neurosci. Rep. 12, 308–317 (2012).
Carelli, V., Ross-Cisneros, F. N. & Sadun, A. A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Retin. Eye Res. 23, 53–89 (2004).
Sadun, A. A., Win, P. H., Ross-Cisneros, F. N., Walker, S. O. & Carelli, V. Leber's hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans. Am. Ophthalmol. Soc. 98, 223–232 (2000).
Bristow, E. A., Griffiths, P. G., Andrews, R. M., Johnson, M. A. & Turnbull, D. M. The distribution of mitochondrial activity in relation to optic nerve structure. Arch. Ophthalmol. 120, 791–796 (2002).
Savini, G. et al. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber's hereditary optic neuropathy mutations. Ophthalmology 112, 127–131 (2005).
Liu, J. & Wang, L.-N. Mitochondrial enhancement for neurodegenerative movement disorders: a systematic review of trials involving creatine, coenzyme Q10, idebenone and mitoquinone. CNS Drugs 28, 63–68 (2014).
Hall, A., Burke, N., Dongworth, R. K. & Hausenloy, D. J. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 171, 1890–1906 (2014).
Yue, W. et al. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 24, 482–496 (2014).
MacLaren, R. E. et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 383, 1129–1137 (2014).
Maresca, A., la Morgia, C., Caporali, L., Valentino, M. L. & Carelli, V. The optic nerve: a “mito-window” on mitochondrial neurodegeneration. Mol. Cell. Neurosci. 55, 62–76 (2013).
Christova, P., Anderson, J. H. & Gomez, C. M. Impaired eye movements in presymptomatic spinocerebellar ataxia type 6. Arch. Neurol. 65, 530–536 (2008).
Tabrizi, S. J. et al. Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 10, 31–42 (2011).
Machuca-Tzili, L., Brook, D. & Hilton-Jones, D. Clinical and molecular aspects of the myotonic dystrophies: a review. Muscle Nerve 32, 1–18 (2005).
Barboni, P. et al. Retinal nerve fiber layer thickness in dominant optic atrophy: Measurements by optical coherence tomography and correlation with age. Ophthalmology 118, 2076–2080 (2011).
Barboni, P. et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber's hereditary optic neuropathy. Ophthalmology 112, 120–126 (2005).
Carelli, V. et al. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim. Biophys. Acta 1787, 518–528 (2009).
Yu-Wai-Man, P., Griffiths, P. G., Hudson, G. & Chinnery, P. F. Inherited mitochondrial optic neuropathies. J. Med. Genet. 46, 145–158 (2009).
Moraes, C. T. et al. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns–Sayre syndrome. N. Engl. J. Med. 320, 1293–1299 (1989).
Grady, J. P. et al. Disease progression in patients with single, large-scale mitochondrial DNA deletions. Brain 137, 323–334 (2014).
Kearns, T. P. & Sayre, G. P. Retinitis pigmentosa, external ophthalmophegia, and complete heart block: unusual syndrome with histologic study in one of two cases. AMA Arch. Ophthalmol. 60, 280–289 (1958).
Rahman, S. et al. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann. Neurol. 39, 343–351 (1996).
Huoponen, K. Leber hereditary optic neuropathy: clinical and molecular genetic findings. Neurogenetics 3, 119–125 (2001).
Finsterer, J. Central nervous system manifestations of mitochondrial disorders. Acta Neurol. Scand. 114, 217–238 (2006).
Tsuji, M. et al. Leigh syndrome associated with West syndrome. Brain Dev. 25, 245–250 (2003).
Morris, A. A. et al. Deficiency of respiratory chain complex I is a common cause of Leigh disease. Ann. Neurol. 40, 25–30 (1996).
Holt, I. J., Harding, A. E., Petty, R. K. & Morgan-Hughes, J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am. J. Hum. Genet. 46, 428–433 (1990).
Sembrano, E., Barthlen, G. M., Wallace, S. & Lamm, C. Polysomnographic findings in a patient with the mitochondrial encephalomyopathy NARP. Neurology 49, 1714–1717 (1997).
Hirano, M. & DiMauro, S. Clinical features of mitochondrial myopathies and encephalomyopathies. In Handbook of Muscle Disease (ed. Lane, R. J.) 479–504 (Marcel Dekker, Inc., 1996).
Hirano, M. & Pavlakis, S. G. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS): current concepts. J. Child Neurol. 9, 4–13 (1994).
Author information
Authors and Affiliations
Contributions
H.M.K. researched the data for the article and wrote the text. All authors made substantial contributions to discussions of the content and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.V.D.-M. has received research grants from Alcon Laboratories, Allergan Limited and the Eye Institute (Auckland, New Zealand). H.M.K and R.H.R. declare no competing interests.
Rights and permissions
About this article
Cite this article
Kersten, H., Roxburgh, R. & Danesh-Meyer, H. Ophthalmic manifestations of inherited neurodegenerative disorders. Nat Rev Neurol 10, 349–362 (2014). https://doi.org/10.1038/nrneurol.2014.79
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2014.79
This article is cited by
-
A Proposal for Classification of Retinal Degeneration in Spinocerebellar Ataxia Type 7
The Cerebellum (2021)
-
Neurophysiological and ophthalmological findings of SPG7-related spastic ataxia: a phenotype study in an Irish cohort
Journal of Neurology (2021)
-
Ocular and neurodevelopmental features of Duchenne muscular dystrophy: a signature of dystrophin function in the central nervous system
European Journal of Human Genetics (2016)
-
Optical coherence tomography findings in Huntington’s disease: a potential biomarker of disease progression
Journal of Neurology (2015)