Abstract
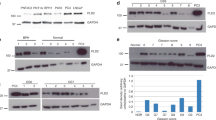
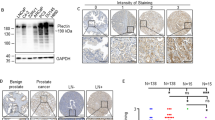
N-acetyl-L-aspartyl-L-glutamate peptidase-like 2 (NAALADL2) is a member of the glutamate carboxypeptidase II family, best characterized by prostate-specific membrane antigen (PSMA/NAALAD1). Using immunohistochemistry (IHC), we have shown overexpression of NAALADL2 in colon and prostate tumours when compared with benign tissue. In prostate cancer, NAALADL2 expression was associated with stage and Grade, as well as circulating mRNA levels of the NAALADL2 gene. Overexpression of NAALADL2 was shown to predict poor survival following radical prostatectomy. In contrast to PSMA/NAALAD1, NAALADL2 was localized at the basal cell surface where it promotes adhesion to extracellular matrix proteins. Using stable knockdown and overexpression cell lines, we have demonstrated NAALADL2-dependent changes in cell migration, invasion and colony-forming potential. Expression arrays of the knockdown and overexpression cell lines have identified nine genes that co-expressed with NAALADL2, which included membrane proteins and genes known to be androgen regulated, including the prostate cancer biomarkers AGR2 and SPON2. Androgen regulation was confirmed in a number of these genes, although NAALADL2 itself was not found to be androgen regulated. NAALADL2 was also found to regulate levels of Ser133 phosphorylated C-AMP-binding protein (CREB), a master regulator of a number of cellular processes involved in cancer development and progression. In combination, these data suggest that changes in expression of NAALADL2 can impact upon a number of pro-oncogenic pathways and processes, making it a useful biomarker for both diagnosis and prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mesters JR, Barinka C, Li W, Tsukamoto T, Majer P, Slusher BS et al. Structure of glutamate carboxypeptidase II, a drug target in neuronal damage and prostate cancer. Embo J 2006; 25: 1375–1384.
Rojas C, Frazier ST, Flanary J, Slusher BS . Kinetics and inhibition of glutamate carboxypeptidase II using a microplate assay. Anal Biochem 2002; 310: 50–54.
Cao KY, Mao XP, Wang DH, Xu L, Yuan GQ, Dai SQ et al. High expression of PSM-E correlated with tumor grade in prostate cancer: a new alternatively spliced variant of prostate-specific membrane antigen. Prostate 2007; 67: 1791–1800.
Liu T, Nedrow-Byers JR, Hopkins MR, Wu LY, Lee J, Reilly PT et al. Targeting prostate cancer cells with a multivalent PSMA inhibitor-guided streptavidin conjugate. Bioorg Med Chem Lett 2012; 22: 3931–3934.
Osborne JR, Akhtar NH, Vallabhajosula S, Anand A, Deh K, Tagawa ST . Prostate-specific membrane antigen-based imaging. Urol Oncol 2012; 31: 144–154.
Barinka C, Mlcochova P, Sacha P, Hilgert I, Majer P, Slusher BS et al. Amino acids at the N- and C-termini of human glutamate carboxypeptidase II are required for enzymatic activity and proper folding. Eur J Biochem 2004; 271: 2782–2790.
Barinka C, Sacha P, Sklenar J, Man P, Bezouska K, Slusher BS et al. Identification of the N-glycosylation sites on glutamate carboxypeptidase II necessary for proteolytic activity. Protein Sci 2004; 13: 1627–1635.
Burgner D, Davila S, Breunis WB, Ng SB, Li Y, Bonnard C et al. A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet 2009; 5: e1000319.
Tonkin ET, Smith M, Eichhorn P, Jones S, Imamwerdi B, Lindsay S et al. A giant novel gene undergoing extensive alternative splicing is severed by a Cornelia de Lange-associated translocation breakpoint at 3q26.3. Human Genet 2004; 115: 139–148.
Statistics OfN. Cancer Statistics Registrations, England 2010.
D'Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Kaplan I et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol 1999; 17: 168–172.
Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT . A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998; 90: 766–771.
Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA 1997; 277: 1445–1451.
Hessels D, Schalken JA . The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol 2009; 6: 255–261.
Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012; 344: d7894.
Augustin H, Mayrhofer K, Pummer K, Mannweiler S . Relationship between prostate cancer gene 3 (PCA3) and characteristics of tumor aggressiveness. Prostate 2012; 73: 203–210.
Botchorishvili G, Matikainen MP, Lilja H . Early prostate-specific antigen changes and the diagnosis and prognosis of prostate cancer. Curr Opin Urol 2009; 19: 221–226.
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18: 11–22.
Bu H, Schweiger MR, Manke T, Wunderlich A, Timmermann B, Kerick M et al. Anterior gradient 2 and 3—two prototype androgen-responsive genes transcriptionally upregulated by androgens and by oestrogens in prostate cancer cells. FEBS J 2013; 280: 1249–1266.
Ho ME, Quek SI, True LD, Morrissey C, Corey E, Vessella RL et al. Prostate cancer cell phenotypes based on AGR2 and CD10 expression. Mod Pathol 2013; 26: 849–859.
Kani K, Malihi PD, Jiang Y, Wang H, Wang Y, Ruderman DL et al. Anterior gradient 2 (AGR2): blood-based biomarker elevated in metastatic prostate cancer associated with the neuroendocrine phenotype. Prostate 2013; 73: 306–315.
Qian X, Li C, Pang B, Xue M, Wang J, Zhou J . Spondin-2 (SPON2), a more prostate-cancer-specific diagnostic biomarker. PLoS One 2012; 7: e37225.
Wayner EA, Quek SI, Ahmad R, Ho ME, Loprieno MA, Zhou Y et al. Development of an ELISA to detect the secreted prostate cancer biomarker AGR2 in voided urine. Prostate 2012; 72: 1023–1034.
Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, Fazli L et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J 2011; 30: 2719–2733.
Varisli L, Gonen-Korkmaz C, Syed HM, Bogurcu N, Debelec-Butuner B, Erbaykent-Tepedelen B et al. Androgen regulated HN1 leads proteosomal degradation of androgen receptor (AR) and negatively influences AR mediated transactivation in prostate cells. Mol Cell Endocrinol 2012; 350: 107–117.
Tsuchiya N, Kamei D, Takano A, Matsui T, Yamada M . Cloning and characterization of a cDNA encoding a novel heterogeneous nuclear ribonucleoprotein-like protein and its expression in myeloid leukemia cells. J Biochem 1998; 123: 499–507.
Conkright MD, Montminy M . CREB: the unindicted cancer co-conspirator. Trends Cell Biol 2005; 15: 457–459.
Barrett JA, Coleman RE, Goldsmith SJ, Vallabhajosula S, Petry NA, Cho S et al. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J Nucl Med 2013; 54: 380–387.
Frigerio B, Fracasso G, Luison E, Cingarlini S, Mortarino M, Coliva A et al. A single-chain fragment against prostate specific membrane antigen as a tool to build theranostic reagents for prostate cancer. Eur J Cancer 2013; S0959-8049: 00087–00087.
Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med 2012; 4: 128ra39.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C . Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997; 3: 81–85.
Tradonsky A, Rubin T, Beck R, Ring B, Seitz R, Mair S . A search for reliable molecular markers of prognosis in prostate cancer: a study of 240 cases. Am J Clin Pathol 2012; 137: 918–930.
Chen HC, Appeddu PA, Isoda H, Guan JL . Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem 1996; 271: 26329–26334.
Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA . Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 2000; 19: 5606–5613.
Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR . Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res 1999; 59: 1655–1664.
Brychtova V, Vojtesek B, Hrstka R . Anterior gradient 2: a novel player in tumor cell biology. Cancer Lett 2011; 304: 1–7.
Wang Z, Hao Y, Lowe AW . The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res 2008; 68: 492–497.
Liao CH, Yeh SC, Huang YH, Chen RN, Tsai MM, Chen WJ et al. Positive regulation of spondin 2 by thyroid hormone is associated with cell migration and invasion. Endocr Relat Cancer 2010; 17: 99–111.
Lee SF, Srinivasan B, Sephton CF, Dries DR, Wang B, Yu C et al. Gamma-secretase-regulated proteolysis of the Notch receptor by mitochondrial intermediate peptidase. J Biol Chem 2011; 286: 27447–27453.
Varisli L, Gonen-Korkmaz C, Debelec-Butuner B, Erbaykent-Tepedelen B, Muhammed HS, Bogurcu N et al. Ubiquitously expressed hematological and neurological expressed 1 downregulates Akt-mediated GSK3beta signaling, and its knockdown results in deregulated G2/M transition in prostate cells. DNA Cell Biol 2011; 30: 419–429.
Powell SM, Brooke GN, Whitaker HC, Reebye V, Gamble SC, Chotai D et al. Mechanisms of androgen receptor repression in prostate cancer. Biochem Soc Trans 2006; 34 (Pt 6): 1124–1127.
Whitaker HC, Kote-Jarai Z, Ross-Adams H, Warren AY, Burge J, George A et al. The rs10993994 risk allele for prostate cancer results in clinically relevant changes in microseminoprotein-beta expression in tissue and urine. PLoS One 2010; 5: e13363.
Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci USA 2004; 101: 10380–10385.
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5: R80.
Cairns JM, Dunning MJ, Ritchie ME, Russell R, Lynch AG . BASH: a tool for managing BeadArray spatial artefacts. Bioinformatics 2008; 24: 2921–2922.
Dunning MJ, Smith ML, Ritchie ME, Tavare S . beadarray: R classes and methods for Illumina bead-based data. Bioinformatics 2007; 23: 2183–2184.
Smyth GK in Limma: Linear Models For Microarray Data (ed. Gentleman VCR, Dudoit S, Irizarry W, Huber R), (Springer, NY, USA, 2005).
Whitaker HC, Stanbury DP, Brinham C, Girling J, Hanrahan S, Totty N et al. Labeling and identification of LNCaP cell surface proteins: a pilot study. Prostate 2007; 67: 943–954.
Thirkettle HJ, Girling J, Warren AY, Mills IG, Sahadevan K, Leung H et al. LYRIC/AEG-1 is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clin Cancer Res 2009; 15: 3003–3013.
Bennett HL, Fleming JT, O'Prey J, Ryan KM, Leung HY . Androgens modulate autophagy and cell death via regulation of the endoplasmic reticulum chaperone glucose-regulated protein 78/BiP in prostate cancer cells. Cell Death Dis 2010; 1: e72.
Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol 2007; 38: 1547–1552.
Tan SS, Ahmad I, Bennett HL, Singh L, Nixon C, Seywright M et al. GRP78 up-regulation is associated with androgen receptor status, Hsp70-Hsp90 client proteins and castrate-resistant prostate cancer. J Pathol 2011; 223: 81–87.
Trojan L, Schaaf A, Steidler A, Haak M, Thalmann G, Knoll T et al. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res 2005; 25: 183–191.
Wu YY, Li H, Lv XY, Wei Q, Li X, Liu XY et al. Overexpression of JKTBP1 induces androgen-independent LNCaP cell proliferation through activation of epidermal growth factor-receptor (EGF-R). Cell Biochem Funct 2008; 26: 467–477.
Acknowledgements
We would like to acknowledge the support of The University of Cambridge, Cancer Research UK, National Medical Research Council, Singapore and Hutchison Whampoa Limited; the National Institute of Health Research, which funds the Cambridge Bio-medical Research Centre, Cambridge, UK; the National Cancer Research Prostate Cancer: mechanisms of Progression and Treatment (PROMPT) collaborative (grant code G0500966/75466), which has funded tissue collections in Cambridge. We would like to thank Prostate Cancer UK for funding a proportion of this work. We are very grateful to the Cancer Research UK Cambridge Institute Genomics Core Facility, Histopathology and ISH Facility, and Bioinformatics and Statistics Core Facility for their assistance with the work in this manuscript. We would also like to thank Erik Sahai (Cancer Research UK London Research Institute) and Ian Mills (Centre for Molecular Medicine Norway) for their helpful discussions in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Whitaker, H., Shiong, L., Kay, J. et al. N-acetyl-L-aspartyl-L-glutamate peptidase-like 2 is overexpressed in cancer and promotes a pro-migratory and pro-metastatic phenotype. Oncogene 33, 5274–5287 (2014). https://doi.org/10.1038/onc.2013.464
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.464
Keywords
This article is cited by
-
Non-invasive molecular imaging of kidney diseases
Nature Reviews Nephrology (2021)
-
Genetic alterations in the 3q26.31-32 locus confer an aggressive prostate cancer phenotype
Communications Biology (2020)
-
HPV16 integration probably contributes to cervical oncogenesis through interrupting tumor suppressor genes and inducing chromosome instability
Journal of Experimental & Clinical Cancer Research (2016)
-
Transcriptome profile of the early stages of breast cancer tumoral spheroids
Scientific Reports (2016)
-
Structural and functional analysis of cell adhesion and nuclear envelope nano-topography in cell death
Scientific Reports (2015)