Abstract
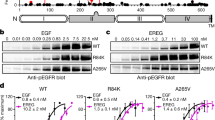
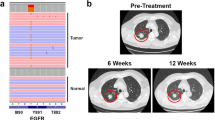
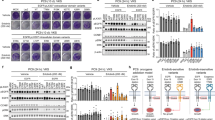
A feature of many gliomas is the amplification of the epidermal growth factor receptor (EGFR), resulting in its overexpression. Missense mutations or deletions within the extracellular domain are associated with this amplification and can lead to constitutive activation of the receptor, with the Domain I/II deletion, EGFRvIII, being the most common. These changes have also been associated with increased sensitivity to EGFR inhibition using small molecule inhibitors. We have expressed, in human glioma cells, EGFR containing four glioma-specific EGFR missense mutations within Domain IV (C620Y, C624F, C628Y and C636Y) to analyze their biological properties and sensitivity to EGFR inhibition. One of these mutants, C620Y, exhibited an enhanced basal phosphorylation, which was partially dependent on an EGFR-ligand autocrine loop. All Domain IV mutants responded equally as well as wildtype EGFR (wtEGFR) to ligand stimulation. Biochemical analysis revealed that a pre-formed, disulfide-bonded dimer associated with these mutations was underglycosylated, inactive and cytoplasmically retained. Ligand stimulation resulted in the formation of a tyrosine-phosphorylated, disulfide-bonded dimer for all Domain IV mutants but not for wtEGFR. Following treatment with the next-generation, irreversible pan-ErbB inhibitor dacomitinib, the C620Y, C624F and EGFRvIII mutants were inactivated, covalently dimerized and were retained in the cytoplasm, resulting in cell-surface receptor loss and, for C620Y and C624F, decreased binding of EGF. Dacomitinib treatment significantly reduced the in vivo growth of human glioma xenografts bearing C620Y, but not wtEGFR. Collectively, these data indicate that the unique biochemical traits of Domain IV EGFR cysteine mutants can be exploited for enhanced sensitivity to EGFR small molecule inhibitors, with potential clinical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 2012; 22: 425–437.
Ymer SI, Greenall SA, Cvrljevic A, Cao DX, Donoghue JF, Epa VC et al. Glioma Specific extracellular missense mutations in the first cysteine rich region of epidermal growth factor receptor (EGFR) initiate ligand independent activation. Cancers 2011; 3: 2032–2049.
Bae JH, Schlessinger J . Asymmetric tyrosine kinase arrangements in activation or autophosphorylation of receptor tyrosine kinases. Mol Cells 2010; 29: 443–448.
Lee JC, Vivanco I, Beroukhim R, Huang JH, Feng WL, DeBiasi RM et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med 2006; 3: e485.
Sharma SV, Bell DW, Settleman J, Haber DA . Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169–181.
Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM . Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Mol Cell Biol 2005; 25: 7734–7742.
Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA . EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell 2003; 11: 507–517.
Arkhipov A, Shan Y, Das R, Endres NF, Eastwood MP, Wemmer DE et al. Architecture and membrane interactions of the EGF receptor. Cell 2013; 152: 557–569.
Frederick L, Wang XY, Eley G, James CD . Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 2000; 60: 1383–1387.
Idbaih A, Aimard J, Boisselier B, Marie Y, Paris S, Criniere E et al. Epidermal growth factor receptor extracellular domain mutations in primary glioblastoma. Neuropathol Appl Neurobiol 2009; 35: 208–213.
Network TCGAR. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068.
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008; 321: 1807–1812.
Mangasarian K, Li Y, Mansukhani A, Basilico C . Mutation associated with Crouzon syndrome causes ligand-independent dimerization and activation of FGF receptor-2. J Cell Physiol 1997; 172: 117–125.
Robertson SC, Meyer AN, Hart KC, Galvin BD, Webster MK, Donoghue DJ . Activating mutations in the extracellular domain of the fibroblast growth factor receptor 2 function by disruption of the disulfide bond in the third immunoglobulin-like domain. Proc Natl Acad Sci USA 1998; 95: 4567–4572.
Watowich SS, Yoshimura A, Longmore GD, Hilton DJ, Yoshimura Y, Lodish HF . Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci USA 1992; 89: 2140–2144.
Yoshimura A, Longmore G, Lodish HF . Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature 1990; 348: 647–649.
Santoro M, Carlomagno F, Romano A, Bottaro DP, Dathan NA, Grieco M et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science 1995; 267: 381–383.
Adams TE, Koziolek EJ, Hoyne PH, Bentley JD, Lu L, Lovrecz G et al. A truncated soluble epidermal growth factor receptor-Fc fusion ligand trap displays anti-tumour activity in vivo. Growth Factors 2009; 27: 141–154.
Johns TG, Mellman I, Cartwright GA, Ritter G, Old LJ, Burgess AW et al. The antitumor monoclonal antibody 806 recognizes a high-mannose form of the EGF receptor that reaches the cell surface when cells over-express the receptor. FASEB J 2005; 19: 780–782.
Cvrljevic AN, Akhavan D, Wu M, Martinello P, Furnari FB, Johnston AJ et al. Activation of Src induces mitochondrial localisation of de2-7EGFR (EGFRvIII) in glioma cells: implications for glucose metabolism. J Cell Sci 2011; 124: 2938–2950.
Bublil EM, Pines G, Patel G, Fruhwirth G, Ng T, Yarden Y . Kinase-mediated quasi-dimers of EGFR. Faseb J 2010; 24: 4744–4755.
Tebbutt N, Pedersen MW, Johns TG . Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer 2013; 13: 663–673.
Fan QW, Cheng CK, Gustafson WC, Charron E, Zipper P, Wong RA et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell 2013; 24: 438–449.
Chong CR, Janne PA . The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013; 19: 1389–1400.
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR et al. The somatic genomic landscape of glioblastoma. Cell 2013; 155: 462–477.
Yu X, Sharma KD, Takahashi T, Iwamoto R, Mekada E . Ligand-independent dimer formation of epidermal growth factor receptor (EGFR) is a step separable from ligand-induced EGFR signaling. Mol Biol Cell 2002; 13: 2547–2557.
He L, Hristova K . Consequences of replacing EGFR juxtamembrane domain with an unstructured sequence. Sci Rep 2012; 2: 854.
Abe Y, Odaka M, Inagaki F, Lax I, Schlessinger J, Kohda D . Disulfide bond structure of human epidermal growth factor receptor. J Biol Chem 1998; 273: 11150–11157.
Macdonald J, Li Z, Su W, Pike LJ . The membrane proximal disulfides of the EGF receptor extracellular domain are required for high affinity binding and signal transduction but do not play a role in the localization of the receptor to lipid rafts. Biochim Biophys Acta 2006; 1763: 870–878.
Li L, Chakraborty S, Yang CR, Hatanpaa KJ, Cipher DJ, Puliyappadamba VT et al. An EGFR wild type-EGFRvIII-HB-EGF feed-forward loop regulates the activation of EGFRvIII. Oncogene (e-pub ahead of print 30 September 2013; doi:10.1038/onc.2013.400).
Ramnarain DB, Park S, Lee DY, Hatanpaa KJ, Scoggin SO, Otu H et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res 2006; 66: 867–874.
Laisney JA, Mueller TD, Schartl M, Meierjohann S . Hyperactivation of constitutively dimerized oncogenic EGF receptors by autocrine loops. Oncogene 2013; 32: 2403–2411.
Lichtner RB, Menrad A, Sommer A, Klar U, Schneider MR . Signaling-inactive epidermal growth factor receptor/ligand complexes in intact carcinoma cells by quinazoline tyrosine kinase inhibitors. Cancer Res 2001; 61: 5790–5795.
Gan HK, Walker F, Burgess AW, Rigopoulos A, Scott AM, Johns TG . The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor AG1478 increases the formation of inactive untethered EGFR dimers. Implications for combination therapy with monoclonal antibody 806. J Biol Chem 2007; 282: 2840–2850.
Arteaga CL, Ramsey TT, Shawver LK, Guyer CA . Unliganded epidermal growth factor receptor dimerization induced by direct interaction of quinazolines with the ATP binding site. J Biol Chem 1997; 272: 23247–23254.
Pillay V, Allaf L, Wilding AL, Donoghue JF, Court NW, Greenall SA et al. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia 2009; 11: 448–458. 2p following 458.
Johns TG, Stockert E, Ritter G, Jungbluth AA, Huang HJ, Cavenee WK et al. Novel monoclonal antibody specific for the de2-7 epidermal growth factor receptor (EGFR) that also recognizes the EGFR expressed in cells containing amplification of the EGFR gene. Int J Cancer 2002; 98: 398–408.
Greenall SA, Gherardi E, Liu Z, Donoghue JF, Vitali AA, Li Q et al. Non-agonistic bivalent antibodies that promote c-MET degradation and inhibit tumor growth and others specific for tumor related c-MET. PLoS ONE 2012; 7: e34658.
Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 2007; 318: 287–290.
Acknowledgements
We would like to thank Professor Antony Burgess from the Walter and Eliza Hall Institute, Melbourne, Victoria for his critical review of the manuscript. SAG is supported by a CSIRO OCE Postdoctoral Fellowship. JFD is funded by a Cure Cancer Australia Foundation Postdoctoral Fellowship. TGJ was supported by NH&MRC project grants (1028552 and 1012020), the Victorian Government’s Operational Infrastructure Support Program and Cure for Life Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
TGJ and NGG received project grant funding from Pfizer.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Greenall, S., Donoghue, J., Gottardo, N. et al. Glioma-specific Domain IV EGFR cysteine mutations promote ligand-induced covalent receptor dimerization and display enhanced sensitivity to dacomitinib in vivo.. Oncogene 34, 1658–1666 (2015). https://doi.org/10.1038/onc.2014.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.106