Abstract
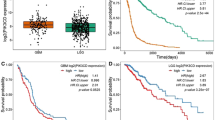
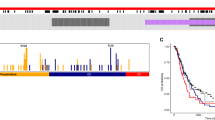
Glioblastoma is the most common and lethal primary malignant brain tumor in adults. The tumor suppressor gene PTEN is deleted, mutated or hypermethylated in more than 60% of glioblastoma cases resulting in hyperactivation of the phosphoinositide 3-kinase pathway, which leads to sustained PI(3,4,5)P3 signaling, and thereby hyperactivation of Akt and other effectors. PI(3,4,5)P3 is also hydrolyzed to PI(3,4)P2 by inositol polyphosphate 5-phosphatases such as SKIP, but the role this pathway has in glioblastoma is unknown. Microarray expression profiling of SKIP in human glioblastoma has revealed both increased and decreased SKIP gene expression. Here we have screened PTEN-deficient glioblastoma for SKIP protein expression by immunohistochemistry and report that SKIP expression is increased in some cases or decreased relative to normal brain. Using the U-87MG PTEN-deficient cell line we show that SKIP knockdown did not further enhance cell proliferation or survival. However, SKIP overexpression in U-87MG cells suppressed anchorage-independent cell growth and growth factor-induced PI(3,4,5)P3/Akt signaling. Although, SKIP knockdown did not affect cell proliferation or survival, cell migration was significantly retarded, associated with significantly increased PI(4,5)P2 signals, and decreased phosphorylation of the actin-regulatory protein cofilin, a PI(4,5)P2-binding protein. Notably, overexpression of SKIP also inhibited migration of U-87MG cells to a similar degree as observed with PTEN reconstitution, however, via distinct mechanisms. PTEN reconstitution promoted sustained lamellipodia generation and focal adhesion formation. In contrast, SKIP overexpression reduced sustained lamellipodia formation, talin incorporation into focal adhesions and recruitment of PI(4,5)P2-binding proteins to the plasma membrane. Notably, analysis of two independent ONCOMINE microarray data sets revealed a significant correlation between increased SKIP mRNA expression in glioblastoma and improved long-term survival. Therefore, SKIP expression in glioblastoma may affect the local invasion of PTEN-deficient tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Filippini G, Falcone C, Boiardi A, Broggi G, Bruzzone MG, Caldiroli D et al. Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol 2008; 10: 79–87.
Rao JS . Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 2003; 3: 489–501.
Franke TF . PI3K/Akt: Getting it right matters. Oncogene 2008; 27: 6473–6488.
Zhang L, Mao YS, Janmey PA, Yin HL . Phosphatidylinositol 4, 5 bisphosphate and the actin cytoskeleton. In: Balla T, Wymann M, York JD (eds) Phosphoinositides II: The Diverse Biological Functions. Springer: The Netherlands, 2012, pp 177–215.
Davies EM, Sheffield DA, Tibarewal P, Fedele CG, Mitchell CA, Leslie NR . The PTEN and Myotubularin phosphoinositide 3-phosphatases: linking lipid signalling to human disease. Subcell Biochem 2012; 58: 281–336.
Koul D . PTEN signaling pathways in glioblastoma. Cancer Biol Ther 2008; 7: 1321–1325.
Beroukhim R, Getz G, Nghiemphu L, Barretina J, Hsueh T, Linhart D et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci USA 2007; 104: 20007–20012.
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006; 9: 391–403.
Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML et al. Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis. Cancer Res 1999; 59: 1820–1824.
Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 2006; 9: 287–300.
Dyson JM, Fedele CG, Davies EM, Becanovic J, Mitchell CA . Phosphoinositide phosphatases: just as important as the kinases. Subcell Biochem 2012; 58: 215–279.
Gurung R, Tan A, Ooms LM, McGrath MJ, Huysmans RD, Munday AD et al. Identification of a novel domain in two mammalian inositol-polyphosphate 5-phosphatases that mediates membrane ruffle localization: the inositol 5-phosphatase SKIP localizes to the endoplasmic reticulum and translocates to membrane ruffles following epidermal growth factor stimulation. J Biol Chem 2003; 278: 11376–11385.
Ijuin T, Mochizuki Y, Fukami K, Funaki M, Asano T, Takenawa T . Identification and characterization of a novel inositol polyphosphate 5- phosphatase. J Biol Chem 2000; 275: 10870–10875.
Ijuin T, Takenawa T . SKIP negatively regulates insulin-induced GLUT4 translocation and membrane ruffle formation. Mol Cell Biol 2003; 23: 1209–1220.
Ijuin T, Takenawa T . Regulation of insulin signaling and glucose transporter 4 (GLUT4) exocytosis by phosphatidylinositol 3,4,5-trisphosphate (PIP3) phosphatase, skeletal muscle, and kidney enriched inositol polyphosphate phosphatase (SKIP). J Biol Chem 2012; 287: 6991–6999.
Ijuin T, Yu YE, Mizutani K, Pao A, Tateya S, Tamori Y et al. Increased insulin action in SKIP heterozygous knockout mice. Mol Cell Biol 2008; 28: 5184–5195.
Schmid AC, Wise HM, Mitchell CA, Nussbaum R, Woscholski R . Type II phosphoinositide 5-phosphatases have unique sensitivities towards fatty acid composition and head group phosphorylation. FEBS Lett 2004; 576: 9–13.
Biegel JA, Burk CD, Barr FG, Emanuel BS . Evidence for a 17p tumor related locus distinct from p53 in pediatric primitive neuroectodermal tumors. Cancer Res 1992; 52: 3391–3395.
Rood BR, Zhang H, Weitman DM, Cogen PH . Hypermethylation of HIC-1 and 17p allelic loss in medulloblastoma. Cancer Res 2002; 62: 3794–3797.
Saxena A, Clark WC, Robertson JT, Ikejiri B, Oldfield EH, Ali IU . Evidence for the involvement of a potential second tumor suppressor gene on chromosome 17 distinct from p53 in malignant astrocytomas. Cancer Res 1992; 52: 6716–6721.
Bredel M, Bredel C, Juric D, Harsh GR, Vogel H, Recht LD et al. High-resolution genome-wide mapping of genetic alterations in human glial brain tumors. Cancer Res 2005; 65: 4088–4096.
Liang Y, Diehn M, Watson N, Bollen AW, Aldape KD, Nicholas MK et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci USA 2005; 102: 5814–5819.
Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF et al. Stem cell-related ‘self-renewal’ signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol 2008; 26: 3015–3024.
Yamanaka R, Arao T, Yajima N, Tsuchiya N, Homma J, Tanaka R et al. Identification of expressed genes characterizing long-term survival in malignant glioma patients. Oncogene 2006; 25: 5994–6002.
Nutt CL, Mani DR, Betensky RA, Tamayo P, Cairncross JG, Ladd C et al. Gene expression-based classification of malignant gliomas correlates better with survival than histological classification. Cancer Res 2003; 63: 1602–1607.
Chalhoub N, Zhu G, Zhu X, Baker SJ . Cell type specificity of PI3K signaling in Pdk1- and Pten-deficient brains. Genes Dev 2009; 23: 1619–1624.
Baeza N, Weller M, Yonekawa Y, Kleihues P, Ohgaki H . PTEN methylation and expression in glioblastomas. Acta Neuropathol 2003; 106: 479–485.
Carico C, Nuño M, Mukherjee D, Elramsisy A, Dantis J, Hu J et al. Loss of PTEN is not associated with poor survival in newly diagnosed glioblastoma patients of the temozolomide era. PLoS ONE 2012; 7: e33684.
Furnari FB, Lin H, Huang HJS, Cavenee WK . Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci USA 1997; 94: 12479–12484.
Steck PA, Pershouse MA, Jasser SA, Yung WKA, Lin H, Ligon AH et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356–362.
Kaplan PL, Anderson M, Ozanne B . Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci USA 1982; 79: 485–489.
King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS . Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol 1997; 17: 4406–4418.
Koopman G, Reutelingsperger CPM, Kuijten GAM, Keehnen RMJ, Pals ST, Van Oers MHJ . Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994; 84: 1415–1420.
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000; 100: 57–70.
Klingler-Hoffmann M, Bukczynska P, Tiganis T . Inhibition of phosphatidylinositol 3-kinase signaling negates the growth advantage imparted by a mutant epidermal growth factor receptor on human glioblastoma cells. Int J Cancer 2003; 105: 331–339.
Li DM, Sun H . PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA 1998; 95: 15406–15411.
Frisch SM, Francis H . Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 1994; 124: 619–626.
Guadamillas MC, Cerezo A, del Pozo MA . Overcoming anoikis—pathways to anchorageindependent growth in cancer. J Cell Sci 2011; 124: 3189–3197.
Davies MA, Lu Y, Sano T, Fang X, Tang P, LaPushin R et al. Adenoviral transgene expression of MMAC/PTEN in human glioma cells inhibits Akt activation and induces anoikis. Cancer Res 1998; 58: 5285–5290.
Koul D, Parthasarathy R, Shen R, Davies MA, Jasser SA, Chintala SK et al. Suppression of matrix metalloproteinase-2 gene expression and invasion in human glioma cells by MMAC/PTEN. Oncogene 2001; 20: 6669–6678.
Tamura M, Gu J, Danen EHJ, Takino T, Miyamoto S, Yamada KM . PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem 1999; 274: 20693–20703.
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE . Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery 2004; 135: 555–562.
Folkman J, Moscona A . Role of cell shape in growth control. Nature 1978; 273: 345–349.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Hinchliffe KA, Irvine RF, Divecha N . Aggregation-dependent, integrin-mediated increases in cytoskeletally associated PtdInsP2 (4,5) levels in human platelets are controlled by translocation of PtdIns 4-P 5-kinase C to the cytoskeleton. EMBO J 1996; 15: 6516–6524.
Guo W, Giancotti FG . Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004; 5: 816–826.
Gu J, Tamura M, Pankov R, Danen EHJ, Takino T, Matsumoto K et al. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J Cell Biol 1999; 146: 389–403.
Tamura M, Gu J, Takino T, Yamada KM . Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130(Cas). Cancer Res 1999; 59: 442–449.
Iijima M, Devreotes P . Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 2002; 109: 599–610.
Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol 2005; 7: 399–404.
Cain RJ, Ridley AJ . Phosphoinositide 3-kinases in cell migration. Biol Cell 2009; 101: 13–29.
Frame M, Norman J . A tal(in) of cell spreading. Nat Cell Biol 2008; 10: 1017–1019.
Mise-Omata S, Obata Y, Iwase S, Mise N, Doi TS . Transient strong reduction of PTEN expression by specific RNAi induces loss of adhesion of the cells. Biochem Biophys Res Commun 2005; 328: 1034–1042.
Várnai P, Bondeva T, Tamás P, Tóth B, Buday L, Hunyady L et al. Selective cellular effects of overexpressed pleckstrin-homology domains that recognize Ptdlns(3,4,5)P3 suggest their interaction with protein binding partners. J Cell Sci 2005; 118: 4879–4888.
Várnai P, Balla T . Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol 1998; 143: 501–510.
Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C . Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol 2008; 87: 649–667.
Gorbatyuk VY, Nosworthy NJ, Robson SA, Bains NPS, Maciejewski MW, dos Remedios CG et al. Mapping the phosphoinositide-binding site on chick cofilin explains how PIP2 regulates the cofilin-actin interaction. Mol Cell 2006; 24: 511–522.
Zhao H, Hakala M, Lappalainen P . ADF/cofilin binds phosphoinositides in a multivalent manner to act as a PIP(2)-density sensor. Biophys J 2010; 98: 2327–2336.
Arber S, Barbayannis FA, Hanser H, Schnelder C, Stanyon CA, Bernards O et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM- kinase. Nature 1998; 393: 805–809.
Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K et al. Cofflin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 1998; 393: 809–812.
Huang S, Lifshitz L, Patki-Kamath V, Tuft R, Fogarty K, Czech MP . Phosphatidylinositol-4,5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes. Mol Cell Biol 2004; 24: 9102–9123.
Kisseleva M, Feng Y, Ward M, Song C, Anderson RA, Longmore GD . The LIM protein Ajuba regulates phosphatidylinositol 4,5-bisphosphate levels in migrating cells through an interaction with and activation of PIPKIα. Mol Cell Biol 2005; 25: 3956–3966.
Sasaki J, Sasaki T, Yamazaki M, Matsuoka K, Taya C, Shitara H et al. Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type I α. J Exp Med 2005; 201: 859–870.
Shibasaki Y, Ishihara H, Kizuki N, Asano T, Oka Y, Yazaki Y . Massive actin polymerization induced by phosphatidylinositol-4-phosphate 5-kinase in vivo. J Biol Chem 1997; 272: 7578–7581.
Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM . Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C δ1. Biochem J 2002; 363: 657–666.
Di Paolo G, De Camilli P . Phosphoinositides in cell regulation and membrane dynamics. Nature 2006; 443: 651–657.
Calderwood DA, Ginsberg MH . Talin forges the links between integrins and actin. Nat Cell Biol 2003; 5: 694–697.
Hynes RO . Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110: 673–687.
Martel V, Racaud-Sultan C, Dupe S, Marie C, Paulhe F, Galmiche A et al. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J Biol Chem 2001; 276: 21217–21227.
Takenawa T, Itoh T . Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta 2001; 1533: 190–206.
Yin HL, Janmey PA . Phosphoinositide regulation of the actin cytoskeleton. Ann Rev Physiol 2003, pp 761–789.
Leslie NR, Yang X, Downes CP, Weijer CJ . PtdIns(3,4,5)P(3)-dependent and -independent roles for PTEN in the control of cell migration. Curr Biol 2007; 17: 115–125.
Liliental J, Moon SY, Lesche R, Mamillapalli R, Li D, Zheng Y et al. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol 2000; 10: 401–404.
Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A . Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 2004; 303: 1179–1181.
Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S et al. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1γ by the FERM domain of talin. Nature 2002; 420: 85–89.
Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA . Type Iγ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 2002; 420: 89–93.
Bertagnolo V, Benedusi M, Brugnoli F, Lanuti P, Marchisio M, Querzoli P et al. Phospholipase C-β2 promotes mitosis and migration of human breast cancer-derived cells. Carcinogenesis 2007; 28: 1638–1645.
Golub T, Caroni P . PI(4/5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J Cell Biol 2005; 169: 151–165.
Campbell RB, Liu F, Ross AH . Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J Biol Chem 2003; 278: 33617–33620.
Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN . Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem 2004; 279: 16606–16613.
Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: 2003–2007.
Abràmoff MD, Magalhães PJ, Ram SJ . Image processing with imageJ. Biophoton Int 2004; 11: 36–41.
Ooms LM, Fedele CG, Astle MV, Ivetac I, Cheung V, Pearson RB et al. The inositol polyphosphate 5-phosphatase, PIPP, is a novel regulator of phosphoinositide 3-kinase-dependent neurite elongation. Mol Biol Cell 2006; 17: 607–622.
Hammond GRV, Schiavo G, Irvine RF . Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P2. Biochem J 2009; 422: 23–35.
Acknowledgements
We thank Dr Tamas Balla (NIH) for kindly providing the GFP-PH/Btk and GFP-PH/PLCδ constructs and Dr Jelena Becanovic for assistance with ONCOMINE data analysis. This work was supported by the National Health & Medical Research Council (NH&MRC), Australia, grant number 384083. We also thank the Biochemistry Imaging Facility, Department of Biochemistry and Molecular Biology, Monash University and the Monash Micro Imaging Facility for technical advice with image acquisition and analysis. Tony Tiganis is a NHMRC research fellow. The results shown here are in part based upon data generated by The Cancer Genome Atlas Research Network http://cancergenome.nih.gov/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Davies, E., Kong, A., Tan, A. et al. Differential SKIP expression in PTEN-deficient glioblastoma regulates cellular proliferation and migration. Oncogene 34, 3711–3727 (2015). https://doi.org/10.1038/onc.2014.303
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.303
This article is cited by
-
Duplicated zebrafish (Danio rerio) inositol phosphatases inpp5ka and inpp5kb diverged in expression pattern and function
Development Genes and Evolution (2023)