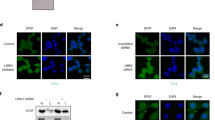
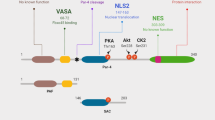
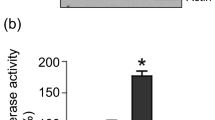
Abstract
Restoration of tumor suppression is an attractive onco-therapeutic approach. It is particularly relevant when a tumor suppressor is excessively degraded by an overactive oncogenic E3 ligase. We previously discovered that the E6-associated protein (E6AP; as classified in the human papilloma virus context) is an E3 ligase that has an important role in the cellular stress response, and it directly targets the tumor-suppressor promyelocytic leukemia protein (PML) for proteasomal degradation. In this study, we have examined the role of the E6AP–PML axis in prostate cancer (PC). We show that knockdown (KD) of E6AP expression attenuates growth of PC cell lines in vitro. We validated this finding in vivo using cell line xenografts, patient-derived xenografts and mouse genetics. We found that KD of E6AP attenuates cancer cell growth by promoting cellular senescence in vivo, which correlates with restoration of tumor suppression by PML. In addition, we show that KD of E6AP sensitizes cells to radiation-induced death. Overall, our findings demonstrate a role for E6AP in the promotion of PC and support E6AP targeting as a novel approach for PC treatment, either alone or in combination with radiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Christian PA, Thorpe JA, Schwarze SR . Velcade sensitizes prostate cancer cells to TRAIL induced apoptosis and suppresses tumor growth in vivo. Cancer Biol Ther 2009; 8: 73–80.
Papandreou CN, Logothetis CJ . Bortezomib as a potential treatment for prostate cancer. Cancer Res 2004; 64: 5036–5043.
Gamell C, Jan Paul P, Haupt Y, Haupt S . PML tumour suppression and beyond: therapeutic implications. FEBS Lett 2014; 588: 2653–2662.
Salomoni P, Ferguson BJ, Wyllie AH, Rich T . New insights into the role of PML in tumour suppression. Cell Res 2008; 18: 622–640.
Wolyniec K, Chan AL, Haupt S, Haupt Y . Restoring PML tumor suppression to combat cancer. Cell Cycle 2012; 11: 3705–3706.
Wolyniec K, Carney DA, Haupt S, Haupt Y . New strategies to direct therapeutic targeting of PML to treat cancers. Front Oncol 2013; 3: 124.
Salomoni P, Pandolfi PP . The role of PML in tumor suppression. Cell 2002; 108: 165–170.
Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP . Identification of a tumour suppressor network opposing nuclear Akt function. Nature 2006; 441: 523–527.
Gurrieri C, Capodieci P, Bernardi R, Scaglioni PP, Nafa K, Rush LJ et al. Loss of the tumor suppressor PML in human cancers of multiple histologic origins. J Natl Cancer Inst 2004; 96: 269–279.
Louria-Hayon I, Alsheich-Bartok O, Levav-Cohen Y, Silberman I, Berger M, Grossman T et al. E6AP promotes the degradation of the PML tumor suppressor. Cell Death Differ 2009; 16: 1156–1166.
Birch SE, Kench JG, Takano E, Chan P, Chan AL, Chiam K et al. Expression of E6AP and PML predicts for prostate cancer progression and cancer-specific death. Ann Oncol 2014; 25: 2392–2397.
Beaudenon S, Huibregtse JM . HPV E6, E6AP and cervical cancer. BMC Biochem 2008; 9: S4.
Srinivasan S, Nawaz Z . E3 ubiquitin protein ligase, E6-associated protein (E6-AP) regulates PI3K-Akt signaling and prostate cell growth. Biochim Biophys Acta 2011; 1809: 119–127.
Khan OY, Fu G, Ismail A, Srinivasan S, Cao X, Tu Y et al. Multifunction steroid receptor coactivator, E6-associated protein, is involved in development of the prostate gland. Mol Endocrinol 2006; 20: 544–559.
Corey E, Quinn JE, Buhler KR, Nelson PS, Macoska JA, True LD et al. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate 2003; 55: 239–246.
Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ . Efficient tumour formation by single human melanoma cells. Nature 2008; 456: 593–598.
Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 1995; 92: 3439–3443.
Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED . The TRAMP mouse as a model for prostate cancer. Curr Protoc Immunol 2001, Chapter 20 Unit 20 25.
Li Z, Chen CJ, Wang JK, Hsia E, Li W, Squires J et al. Neuroendocrine differentiation of prostate cancer. Asian J Androl 2013; 15: 328–332.
Wolyniec K, Shortt J, de Stanchina E, Levav-Cohen Y, Alsheich-Bartok O, Louria-Hayon I et al. E6AP ubiquitin ligase regulates PML-induced senescence in Myc-driven lymphomagenesis. Blood 2012; 120: 822–832.
de The H, Le Bras M, Lallemand-Breitenbach V . The cell biology of disease: acute promyelocytic leukemia, arsenic, and PML bodies. J Cell Biol 2012; 198: 11–21.
Steiner MS, Wang Y, Zhang Y, Zhang X, Lu Y . p16/MTS1/INK4A suppresses prostate cancer by both pRb dependent and independent pathways. Oncogene 2000; 19: 1297–1306.
Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985; 318: 533–538.
Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 2003; 4: 223–238.
Ivanschitz L, De The H, Le Bras M . PML, SUMOylation, and senescence. Front Oncol 2013; 3: 171.
Oh W, Ghim J, Lee EW, Yang MR, Kim ET, Ahn JH et al. PML-IV functions as a negative regulator of telomerase by interacting with TERT. J Cell Sci 2009; 122: 2613–2622.
Mallette FA, Goumard S, Gaumont-Leclerc MF, Moiseeva O, Ferbeyre G . Human fibroblasts require the Rb family of tumor suppressors, but not p53, for PML-induced senescence. Oncogene 2004; 23: 91–99.
Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest 2010; 120: 681–693.
Pernicova Z, Slabakova E, Kharaishvili G, Bouchal J, Kral M, Kunicka Z et al. Androgen depletion induces senescence in prostate cancer cells through down-regulation of Skp2. Neoplasia 2011; 13: 526–536.
Quignon F, De Bels F, Koken M, Feunteun J, Ameisen JC, de The H . PML induces a novel caspase-independent death process. Nat Genet 1998; 20: 259–265.
Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol 2004; 22: 2108–2121.
Kraft AS, Garrett-Mayer E, Wahlquist AE, Golshayan A, Chen CS, Butler W et al. Combination therapy of recurrent prostate cancer with the proteasome inhibitor bortezomib plus hormone blockade. Cancer Biol Ther 2011; 12: 119–124.
Dreicer R, Petrylak D, Agus D, Webb I, Roth B . Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin Cancer Res 2007; 13: 1208–1215.
Price N, Dreicer R . Phase I/II trial of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin Prostate Cancer 2004; 3: 141–143.
Yamagishi Y, Shoji I, Miyagawa S, Kawakami T, Katoh T, Goto Y et al. Natural product-like macrocyclic N-methyl-peptide inhibitors against a ubiquitin ligase uncovered from a ribosome-expressed de novo library. Chem Biol 2011; 18: 1562–1570.
Chen RH, Lee YR, Yuan WC . The role of PML ubiquitination in human malignancies. J Biomed Sci 2012; 19: 81.
Herold MJ, van den Brandt J, Seibler J, Reichardt HM . Inducible and reversible gene silencing by stable integration of an shRNA-encoding lentivirus in transgenic rats. Proc Natl Acad Sci USA 2008; 105: 18507–18512.
Levav-Cohen Y, Wolyniec K, Alsheich-Bartok O, Chan AL, Woods SJ, Jiang YH et al. E6AP is required for replicative and oncogene-induced senescence in mouse embryo fibroblasts. Oncogene 2012; 31: 2199–2209.
Acknowledgements
This work was supported by NHMRC project grants (1049179 and 1063389) and NHMRC Fellowship to YH (9628426), NHMRC project grant to MJH (1049720), PCF creativity grant to SW and YH, CCV grant (1085154), VCA Richard Pratt Fellowship in Prostate Cancer Research to CG, the PCFA grant (NDDA-1811) to EW, Pfizer Australia, NHMRC and Veski foundings to MS, and a prostate initiative grant from the Foundation of the Peter MacCallum Cancer Centre. PrEC line was a generous gift from Dr Patrick Humbert and Helen Pearson at Peter MacCallum Cancer Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Paul, P., Raghu, D., Chan, AL. et al. Restoration of tumor suppression in prostate cancer by targeting the E3 ligase E6AP. Oncogene 35, 6235–6245 (2016). https://doi.org/10.1038/onc.2016.159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.159
This article is cited by
-
Using the Microwell-mesh to culture microtissues in vitro and as a carrier to implant microtissues in vivo into mice
Scientific Reports (2021)
-
The role of E3 ubiquitin ligase HECTD3 in cancer and beyond
Cellular and Molecular Life Sciences (2020)
-
E3 ubiquitin ligases in cancer and implications for therapies
Cancer and Metastasis Reviews (2017)