Abstract
Background:
Our aim is to study the prevalence of subclinical celiac disease (CD) and analyze the diagnostic yield of a new rapid test in children aged 2–4.
Methods:
We carried out a cross-sectional study in a sample population of children aged 2–4 from the same metropolitan area. We recruited apparently healthy subjects, and collected clinical, anthropometric, analytical, and serological variables. We also tested for anti-gliadin IgA and anti-transglutaminase IgG and IgA using a rapid immunochromatographic test CD1WB and CD2WB (Operon, Zaragoza, Spain).
Results:
One hundred and ninety-eight children were recruited, signed the informed consent form, and completed the protocol (mean age 32.3 ± 9.2 mo, 53% males). CD prevalence according to the serological tests was 3% (CI 95%, 1.4–6.4%). Biopsies were used to confirm the diagnosis in all suspected cases. The sensitivity and negative predictive value of the CD2WB immunochromatographic test strip were 100% and 1, respectively. The sensitivity of CD1WB was 16.6% and its specificity was high (89.1%).
Conclusion:
The prevalence of subclinical CD in the sample group of 2–4-y old was higher than that found by other authors. The CD2WB immunochromatographic test strip is an excellent diagnostic screening tool with high sensitivity and negative predictive value.
Similar content being viewed by others
Main
Celiac disease (CD) is a world immune-mediated disorder caused by a reaction to the gliadins in the gluten in many common foodstuffs. It affects those with a genetic predisposition to the disease and is the most prevalent cause of digestive malabsorption in the developed world. CD symptoms can range from very severe to subclinical. It is estimated that for every diagnosed case of CD there are five undetected cases. The estimated global prevalence of CD is between 1 in 100 and 1 in 250, although few specific data about our study population of 2–4-y olds are available (1,2,3,4).
Early detection of CD is a challenge for all pediatricians. Current diagnostic methods include serological tests to detect autoantibodies and histological tests following upper gastrointestinal endoscopy and biopsy of the intestinal villi, although both methods are invasive in certain degree (5). The high estimated prevalence of subclinical CD in apparently healthy children means that a noninvasive screening method is required. The results of previous studies using a rapid visual immunochromatographic bedsite test (point-of-care testing) have been promising (6,7,8,9,10,11,12,13).
Our aim is to study the prevalence of subclinical CD in children aged 2–4 living in the same Healthcare District (Maracena, in the metropolitan district of Granada, Spain), and to assess the validity of an immunochromatographic screening method that detects CD antibodies in the capillary blood of apparently healthy subjects (Operon).
Results
Two hundred and fifty-two children aged between 2 and 4 at the time of recruitment were included in the study group between May 2009 and April 2012. The final sample size was n = 198 children (average age 32.3 ± 9.2 mo, 53% male). All of these children completed the study protocol. The group’s clinical and sociodemographic variables are shown in Table 1 .
Anthropometric and nutritional variables for the 198 subjects were collected to establish how similar the study group was to the general population. All the average percentiles calculated were similar to those of the general population.
Prevalence of CD
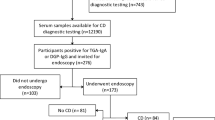
Figure 1a is a flow diagram showing the process by which children were included in the study. Of the 198 children tested using the point-of-care test and the auto-antibody test using EliA (Enzyme immunoassay, Phadia, Uppsala, Sweden) or secondary immunofluorescence, 27 tested positive with at least one of the capillary blood test strips. In six of those cases, the serological test was also positive and the diagnosis was confirmed with a biopsy. In 21 cases, the serological tests did not confirm the CD diagnosis.
Results. (a) Flow chart of study. (b) Prevalence of subclinical celiac disease (CD) of 3%. (c) Diagnostic validity of CD1WB and CD2WB test strips in positive cases. (d) Diagnostic validity of CD1WB and CD2WB test strips in negative cases. Note the very low proportion of false negatives for both strips. Cases with CD are represented in black in panels b–d, and healthy children are represented in gray.
Of the six children with a positive anti-transglutaminase and anti-endomysial antibody test, in five cases (83.3%) the biopsy confirmed CD with a Marsh score of 3 ( Figure 2a ). In one case, the biopsy revealed no signs of villus atrophy in the duodenal mucosa, with raised intraepithelial lymphocytes (>30) and crypt hyperplasia consistent with a Marsh score of 2. In another case, the patient tested positive with the test strips and the serological test results were consistent with dermatitis herpetiformis associated with CD ( Figure 2b ). During the diagnostic procedure, this child developed a pruritic rash on the back, buttocks, and elbows but had experienced no digestive symptoms.
Diagnosis of CD in two cases (a) Case 2 biopsy with hematoxylin and eosin stain showing villus atrophy (10× magnification in both panels). (b) Case 5, a 41-mo-old male diagnosed with dermatitis herpetiformis using secondary immunofluorescence; note IgA acanthocytes on monkey oesophagus substrate (40× magnification on the left panel, and 20× on the right panel).
Based on the results of the serological tests for anti-transglutaminase and anti-endomysial IgA antibodies and the biopsies, the prevalence of CD in the sample group was 3% (CI 95%, 1.4–6.4%, Figure 1b ).
The average age of patients with CD (n = 6) was 36.1 ± 10.1 mo. Half of them were male. All were initially asymptomatic. Table 2 shows the variables for the six patients initially diagnosed with CD based on the serological tests and whose diagnoses were later confirmed histologically.
The clinical and sociodemographic variables and family history of the children with CD were no different to those of the 192 healthy subjects (P > 0.05). There were no significant differences in the digestive symptoms of the group with CD and the group without, with very similar percentages for the frequency of abdominal pain, vomiting, and anorexia, among others.
The analytical and anthropometric variables for the patients with and without CD are shown in Table 3 . The blood glucose, creatinine, urea, and electrolyte results were not different between the two groups. However, the total cholesterol and albumin levels were lower in the group with CD (statistically significant, P < 0.05). The weight and height-for-age percentiles were within the normal range according to the percentile charts used (11), with no statistically significant differences between the six patients with CD and the 192 without. The height-for-age percentile comparison was not statistically significant, but the data suggest that patients with subclinical CD are more likely to be shorter than average.
Analysis of the Diagnostic Validity of the Point-of-Care Test (Operon)
As Figure 1c , d shows, 27 of the subjects tested positive for CD with at least one of the test strips used. Of these, all tested positive with the CD2WB strip except 1, who had a weak positive result with the CD1WB strip and was eventually confirmed not to have CD. All of the remaining 171 subjects tested negative with both test strips. None of these 171 patients tested positive with the serological tests and there was no confirmed case of CD. The sensitivity of CD2WB alone and both strips together is therefore 100%.
Figure 1c shows the distribution of cases with positive results with the test strips. Of the three subjects who tested positive with CD1WB, only one was later confirmed to have CD. Similarly, of the 27 initially positive tests with CD2WB, only 6 were later confirmed to be CD. The positive predictive value of both tests for diagnosing CD was therefore limited in this study population where CD prevalence was low (3%).
However, the analysis of cases with negative capillary blood test results revealed interesting findings in terms of the sensitivity and negative predictive value of the strips. These are shown in Figure 1d . The negative predictive value of both test strips is high; in other words, the likelihood of a patient having CD when the capillary test is negative is very low. The percentages of true positives and negatives, false positives and negatives, sensitivity, and specificity are shown in Table 4 .
Discussion
CD is a large-scale health problem and could be considered one of the most frequent genetic autoimmune diseases in the Western world. CD’s high prevalence, its associated comorbidities in the short and long term, and the fact that an effective treatment is available mean that the disease is a good candidate for a screening program (4,14). Screening would not only detect “subclinical” CD patients but also atypical patients who are difficult to diagnose because of their nonspecific symptoms (15,16,17). Despite this, previous studies have argued against screening for CD because “asymptomatic” patients may not adhere to a gluten-free diet (18). We have assessed a new technique for detecting subclinical CD by testing for specific antibodies using a visual immunochromatographic test kit. The kit tests capillary blood, providing a quick and simple diagnosis at the point of care, making it suitable for use in primary care centers (19,20). Point-of-care tests are quick to use, inexpensive, and not technically complex. They also offer a further advantage over other tests studied previously, in that serum IgA does not need to be measured, and the test can therefore be used in patients with an IgA deficiency, a frequent condition in patients under 3 y of age. The test can be performed easily by pediatricians or general practitioners.
The high sensitivity of the CD2WB test found here allows us to conclude that it is an excellent screening method, guaranteeing detection of CD in all patients. However, the data should be interpreted carefully, as although the sensitivity and negative predictive value of the CD2WB test strip were both 100%, the negative predictive value of the CD1WB strip was 97.4%. This is due more to the low prevalence of CD in the population analyzed than to the value of the test itself, which had a sensitivity of just 16.6%. As the overall prevalence of CD in the sample group was 3%, the positive predictive value could be expected to be low (0.22), and more specific tests would be required in order to confirm the diagnosis. According to the data collected here, if a patient tests positive with the CD2WB strip, their likelihood of having CD increases from 3 to 22% (likelihood ratio positive 9); if the patient tests negative, their likelihood of having CD does not change (likelihood ratio negative 0.8).
It is very interesting that the study population consisted of asymptomatic subjects who would never have been tested for CD basing on their clinical symptoms. They were only diagnosed and a gluten-free diet prescribed at an early stage of the disease thanks to the new screening method studied. Previous studies have assessed the same kit (Operon) or other similar methods (Biocard, ANI Biotech, Finland) in subjects with CD, suspected CD or with close family members with CD, i.e., groups with a higher prevalence of CD. In all of those studies the positive predictive value of the test was much higher than the value found here, and the sensitivity results were also close to 100%. However, our study supports previous evidence to demonstrate the real value of this test kit as a CD screening method in asymptomatic subjects (12).
The prevalence of CD in our study group of apparently healthy patients suggests that 1 in 33 people have subclinical CD. This is much higher than the prevalences found in previous studies, and higher than expected. This high prevalence is even more significant given the age of the study population (2–4 y) and the lack of initial symptoms in the cohort. To date, the highest prevalence in a healthy, asymptomatic Spanish population was found by García Novo (1 in 220), and higher prevalences have only been found by Maki et al. in Finland (1 in 98), and in studies in a Saharawi population (1 in 18) (1,2,3,4,21,22,23,24,25,26). The sample group of patients with CD here was small, so it is impossible to draw reliable conclusions about their clinical, analytical, or nutritional status. However, the results suggest that CD patients tend to have early biochemical markers of malnutrition, such as low albumin levels and smaller subscapular skinfolds.
Conclusion
The prevalence of subclinical CD found in the sample group of children aged 2–4 was higher than the prevalences found in previous studies. The clinical, analytical, and nutritional variables for patients with and without CD were similar.
The sensitivity and negative predictive value of the CD2WB immunochromatographic test strips in the sample group were 100% and 1, respectively. The sensitivity of the CD1WB test strip was 16.6%. In conclusion, the CD2WB test strip provides excellent diagnostic validity as a screening test to detect subclinical CD.
Methods
Children between 2 and 4 y of age from the same Healthcare District (Maracena, Granada) were recruited into a prospective cohort between May 2009 and April 2012. Two hundred and fifty-two subjects were selected using a computer-generated random sequence. Informed consent forms were signed by the parents or guardians of 198 of the subjects, who were therefore recruited. The inclusion criteria required an absence of symptoms suggestive of CD at the time of recruitment; so, patients with suspected CD or a confirmed diagnosis were excluded. Patients with minor, sporadic symptoms, and in no case suggestive of CD were not excluded. This study was approved by the Granada (Spain) Healthcare District’s Ethics Committee and Virgen de las Nieves Universitary Hospital Ethics Committee, and an informed consent form was signed by the subject’s parents or guardians in all participants.
Data Collection
All of the patients underwent a clinical examination and their clinical, anthropometric, and nutritional variables were collected. During the same appointment, rapid test kids manufactured by Operon were used to detect immune markers in the subjects’ capillary blood serum. These kits consist of the following two test strips: (i) SIMPLE-CD1WB: detects antibodies (IgA/IgG/IgM) against human tissue transglutaminase. (ii) SIMPLE-CD2WB: detects IgA-type antibodies against human tissue transglutaminase and antibodies against gliadins.
Whatever the results of those rapid tests, the following data were then collected for all subjects: complete blood count, blood chemistry, nutritional variables, and growth scores (including weight, height, arm circumference, and subscapular, triceps, and biceps skinfold measurements). Serological tests for CD antibodies were also performed: anti-tissue transglutaminase IgA and IgG (tTG IgA and tTG IgG) and deamidated gliadin peptide (DGP-IgA and IgG), using EliA Celikey (IgA kit Phadia, Uppsala, Sweden), and anti-endomysial antibodies (EMA-IgA) using secondary immunofluorescence, in accordance with the manufacturer guidelines (Thermo Scientific, Phadia for TTG with a cut-off value for IgA and IgG 0–10 U/ml and DiaMedix, (Miami, FL) for antiendomysial antibodies (EMA) using monkey distal esophagus substrate with a cut-off value 1/5 dilution).
In cases where CD was thought highly likely, an upper gastrointestinal endoscopy was performed and biopsies taken for histological analysis, including bulb biopsies from the duodenum.
Statistical Analysis
The statistical analysis was carried out using SPSS 17.0 (Chicago). In order to study the prevalence of subclinical CD, descriptive measures were used calculating a CI of 95%. The clinical, analytical, and anthropometric variables of subjects with CD were analyzed by calculating central tendency (mean and median) and dispersion (SD). These measures were compared with those of the group without CD using the Student’s t-test and Fisher’s exact test. The level of significance was set at P < 0.05.
In order to assess the validity of the new immunochromatographic diagnostic test, a cross-sectional analysis was carried out using the detection of anti-transglutaminase and anti-endomysial antibodies as the gold standard. The proportions of true and false positives and negatives were calculated. These were then used to calculate the positive and negative predictive values and specificity of the new test.
Statement of Financial Support
José Maldonado and Rosario Moreno-Torres received a grant from the “Sistema Andaluz de Salud” (Andalusian Health System).
Disclosure
none were declared.
References
Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 2003;163:286–92.
Mustalahti K, Catassi C, Reunanen A, et al.; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 2010;42:587–95.
Mäki M, Mustalahti K, Kokkonen J, et al. Prevalence of Celiac disease among children in Finland. N Engl J Med 2003;348:2517–24.
Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics 2013;131:e687–94.
Husby S, Koletzko S, Korponay-Szabó IR, et al.; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–60.
Korponay-Szabó IR, Kovács JB, Czinner A, Gorácz G, Vámos A, Szabó T. High prevalence of silent celiac disease in preschool children screened with IgA/IgG antiendomysium antibodies. J Pediatr Gastroenterol Nutr 1999;28:26–30.
Korponay-Szabó IR, Raivio T, Laurila K, et al. Coeliac disease case finding and diet monitoring by point-of-care testing. Aliment Pharmacol Ther 2005;22:729–37.
Raivio T, Kaukinen K, Nemes E, et al. Self transglutaminase-based rapid coeliac disease antibody detection by a lateral flow method. Aliment Pharmacol Ther 2006;24:147–54.
Raivio T, Korponay-Szabó I, Collin P, et al. Performance of a new rapid whole blood coeliac test in adult patients with low prevalence of endomysial antibodies. Dig Liver Dis 2007;39:1057–63.
Baldas V, Tommasini A, Trevisiol C, et al. Development of a novel rapid non-invasive screening test for coeliac disease. Gut 2000;47:628–31.
Baviera LC, Aliaga ED, Ortigosa L, et al. Celiac disease screening by immunochromatographic visual assays: results of a multicenter study. J Pediatr Gastroenterol Nutr 2007;45:546–50.
Korponay-Szabó IR, Szabados K, Pusztai J, et al. Population screening for coeliac disease in primary care by district nurses using a rapid antibody test: diagnostic accuracy and feasibility study. BMJ 2007;335:1244–7.
Nenna R, Tiberti C, Petrarca L, et al. The celiac iceberg: characterization of the disease in primary schoolchildren. J Pediatr Gastroenterol Nutr 2013;56:416–21.
Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology 2001;120:636–51.
Roma E, Panayiotou J, Karantana H, et al. Changing pattern in the clinical presentation of pediatric celiac disease: a 30-year study. Digestion 2009;80:185–91.
Garampazzi A, Rapa A, Mura S, et al. Clinical pattern of celiac disease is still changing. J Pediatr Gastroenterol Nutr 2007;45:611–4.
Corazza GR, Frisoni M, Treggiari EA, et al. Subclinical celiac sprue. Increasing occurrence and clues to its diagnosis. J Clin Gastroenterol 1993;16:16–21.
Fabiani E, Taccari LM, Rätsch IM, Di Giuseppe S, Coppa GV, Catassi C. Compliance with gluten-free diet in adolescents with screening-detected celiac disease: a 5-year follow-up study. J Pediatr 2000;136:841–3.
Shamir R, Yehezkely-Schildkraut V, Hartman C, Eliakim R. Population screening for celiac disease: follow up of patients identified by positive serology. J Gastroenterol Hepatol 2007;22:532–5.
Demirçeken FG, Kansu A, Kuloğlu Z, Girgin N, Güriz H, Ensari A. Human tissue transglutaminase antibody screening by immunochromatographic line immunoassay for early diagnosis of celiac disease in Turkish children. Turk J Gastroenterol 2008;19:14–21.
Ferre-López S, Ribes-Koninckx C, Genzor C, et al. Immunochromatographic sticks for tissue transglutaminase and antigliadin antibody screening in celiac disease. Clin Gastroenterol Hepatol 2004;2:480–4.
Dubé C, Rostom A, Sy R, et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology 2005;128(4 Suppl 1):S57–67.
Ascher H, Krantz I, Kristiansson B. Increasing incidence of coeliac disease in Sweden. Arch Dis Child 1991;66:608–11.
Vitoria Cormenzana JC, Sojo Aguirre A, Martín Bejarano E, Zuazo Zamalloa E, Corera Sánchez M, Escudero Jiménez F. [Incidence of celiac disease in Vizcaya]. An Esp Pediatr 1991;35:251–3.
García Novo MD, Garfia C, Acuña Quirós MD, et al. [Prevalence of celiac disease in apparently healthy blood donors in the autonomous community of Madrid]. Rev Esp Enferm Dig 2007;99:337–42.
Riestra S, Fernández E, Rodrigo L, Garcia S, Ocio G. Prevalence of Coeliac disease in the general population of northern Spain. Strategies of serologic screening. Scand J Gastroenterol 2000;35:398–402.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Almazán, M., Ortega, E., Moreno Torres, R. et al. Diagnostic screening for subclinical celiac disease using a rapid test in children aged 2–4. Pediatr Res 78, 280–285 (2015). https://doi.org/10.1038/pr.2015.98
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.98
This article is cited by
-
Office-Based Point of Care Testing (IgA/IgG-Deamidated Gliadin Peptide) for Celiac Disease
American Journal of Gastroenterology (2018)