Abstract
Fetal growth restriction (FGR) is a common complication of pregnancy and, in severe cases, is associated with elevated rates of perinatal mortality, neonatal morbidity, and poor neurodevelopmental outcomes. The leading cause of FGR is placental insufficiency, with the placenta failing to adequately meet the increasing oxygen and nutritional needs of the growing fetus with advancing gestation. The resultant chronic fetal hypoxia induces a decrease in fetal growth, and a redistribution of blood flow preferentially to the brain. However, this adaptation does not ensure normal brain development. Early detection of brain injury in FGR, allowing for the prediction of short- and long-term neurodevelopmental consequences, remains a significant challenge. Furthermore, in FGR infants the detection and diagnosis of neuropathology is complicated by preterm birth, the etiological heterogeneity of FGR, timing of onset of growth restriction, its severity, and coexisting complications. In this review, we examine existing and emerging diagnostic tools from human and preclinical studies for the detection and assessment of brain injury in FGR fetuses and neonates. Increased detection rates, and early detection of brain injury associated with FGR, will offer opportunities for developing and assessing interventions to improve long-term outcomes.
Similar content being viewed by others
Main
Fetal growth restriction (FGR) or intrauterine growth restriction (IUGR) affects more than 10% of pregnancies worldwide, with substantial implications for short-term and long-term well-being of the infant. FGR is strongly associated with stillbirth, preterm birth, and, in newborn survivors, increased risk of developing neonatal complications (1). FGR is also a causal factor in the development of adverse neurodevelopmental sequelae in childhood (2, 3).
FGR defines a fetus that has failed to reach its genetically determined birth weight. Unfortunately, there is a lack of a consensus definition for fetal growth restriction. Pragmatically, FGR is defined by the criteria of estimated fetal weight, or birth weight, being less than the 10th centile for age and sex. However, many studies do not discriminate between infants whose birth weight is less than the 10th centile for age but who are small and otherwise healthy, termed small for gestational age (SGA), compared with the pathologically small babies who did not grow fully (true FGR). Further, some fetuses will be growth-restricted, but have a birth weight >10th centile. Such fetuses remain at an increased risk of stillbirth or perinatal morbidity.
The causes of FGR are diverse, including fetal, maternal, or placental factors (4). Poor placental function is the most important contributor clinically (5, 6, 7), resulting in chronic fetal hypoxia and hypoglycemia in an otherwise normal fetus (8, 9, 10). In turn, chronic fetal hypoxemia and nutrient insufficiency directly decrease fetal growth rate, and hypoxia induces a redistribution of cardiac output (11, 12, 13, 14). This redistribution of fetal cardiac output tends to protect brain and heart growth relative to other organs, termed brain-sparing or central redistribution, but this does not ensure normal brain growth (4, 9, 15).
The specific neuropathology of FGR is complex and distinct from that in both infants born preterm without FGR and in term infants exposed to a severe acute hypoxic event (2, 16). Human FGR imaging studies and postmortem examination, together with animal experimental studies of placental insufficiency and FGR, describe reduced total brain volume, with loss of both gray and white matter substructure. At the cellular level, gray matter areas are shown to have reduced cell number (17) with sparse and disorganized cortical structure (18). The white matter of the FGR brain is described as immature, with delayed oligodendrocyte maturation (19), more unmyelinated axons, and thinner myelin coverage (20), with evidence of astrogliosis and inflammation (21). More recently, it has also been shown that the structural connectivity of the FGR brain is significantly altered, particularly along motor and cortico-striatal-thalamic tracts. Importantly, these measures of reduced tractography correlate with poor neurodevelopmental outcomes in young children who were born FGR (22, 23).
FGR is associated with an increased risk for neurodevelopmental impairment, with the degree of impairment related to (i) the severity of growth restriction, (ii) the onset of FGR (early or late), and (iii) gestation at birth (preterm or term) (2). FGR children born preterm or with evidence of brain-sparing are considered to be at greatest risk for deficits in brain development (24). The neurodevelopmental outcomes of children born after early-onset FGR are worse than outcomes for late-onset FGR. This likely reflects both a greater degree of placental dysfunction and hypoxia adversely affecting brain development, and the impact of preterm birth (25). In addition, preterm FGR infants demonstrate an elevated risk for neonatal complications such as pulmonary hypertension, metabolic disturbances, and necrotizing enterocolitis, which in turn may induce acute hypoxia/ischemia leading to increased brain injury (26). Late-onset FGR infants are also at risk for altered outcomes, particularly infants with brain-sparing, who show abnormal neurobehavior in the neonatal period and at 2 years of age (27, 28, 29). Impairments in school-age children who have FGR encompass gross and fine motor deficits, cognition and learning problems, and behavioral dysfunctions (30, 31), and neurological dysfunctions continue into older childhood and adolescence (32). Furthermore, FGR is associated with high risk for diagnosis of cerebral palsy. The rate of cerebral palsy for early-onset FGR is up to 12% for infants delivered at <32 weeks of gestation (25, 33, 34).
The complex and heterogeneous adverse outcomes observed in FGR children demonstrate the need for accurate neurological assessments that can be applied either antenatally or postnatally, and for the provision of a diagnostic link between the injury observed and long-term consequences. The objective of this review is to bring together the available evidence for the detection and assessment of brain injury linked to FGR, in both the fetus and neonate. We acknowledge that, with no strict definition for FGR, this is imperfect; however, here we have only included published work in which the population was described as “FGR” or “IUGR”.
Assessment of fgr-related brain injury in the fetus
Fetal Ultrasound
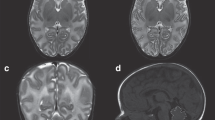
Ultrasound-based fetal surveillance is an established component of modern perinatal care of high-risk pregnancy, including the monitoring of FGR (Figures 1 and 2) (35, 36, 37). A major aspect of assessment of fetal well-being and, indirectly, neuropathology in FGR relies on Doppler assessment of fetal and uteroplacental circulations. As placental pathology is considered the principal cause of true FGR (7, 10), recent definitions of FGR include fetal umbilical artery Doppler flow velocimetry assessment (36, 38). However, it is suggested that a definition of true FGR should not rely on parameters of fetal umbilical artery Doppler alone, as this parameter identifies only severe, early-onset placental insufficiency (39). Instead, FGR should be diagnosed by the presence of poor fetal growth combined with any Doppler observation associated with suboptimal perinatal outcome in umbilical or uterine artery (UA), or cerebral arteries. A recent study in a cohort of FGR fetuses confirmed that evaluation of Doppler parameters, rather than gestational age at birth, allowed better risk stratification of FGR preterm fetuses for neonatal neuropathologies (40).
Fetal ultrasound—umbilical artery Doppler
Growth-restricted fetuses with absent or reversed end-diastolic flow in the UA have increased rates of fetal and neonatal mortality, and a higher incidence of long-term permanent neurologic damage (41). The importance of following UA Doppler status is demonstrated particularly in early-onset FGR, where end-diastolic velocity is reversed in the UA or aorta, whereas cerebral vascular impedance changes are apparent in both early- and late-onset FGR (38). Absent diastolic flow in the UA is a sign of placental resistance and vascular stress, with increasing placental resistance leading to reversal of flow in an already compromised fetal placental unit. Fetal Doppler indices, such as absent or reversed end-diastolic flow in the UA and absent or reversed “a” wave in the ductus venosus (DV), are considered good predictors of neonatal intraventricular hemorrhage (IVH) and death in growth-restricted infants (42, 43). Infants who demonstrate an altered UA have poorer motor outcomes at 2 years and at school age when compared with their appropriately grown preterm or term counterparts (44, 45). It is pertinent to note that the detection of placental and fetal circulatory abnormalities via ultrasound does not provide a direct assessment of brain injury but, along with fetal head circumference, severity of growth restriction, and gestational age at delivery, they are very useful determinants of the degree of placental dysfunction, which is, in turn, associated with neurodevelopmental outcomes (46). The overall sensitivity and specificity of reverse end-diastolic flow in UA or DV and adverse perinatal outcomes is 60–80%, with a positive predictive value (PPV) of ~50% and negative predictive value (NPV) of 80% (47, 48).
Fetal ultrasound—cerebral doppler
Assessment of the fetal cerebral circulation is particularly useful to observe hemodynamic changes associated with chronic hypoxia and the severity of FGR. The gold standard for fetal brain hemodynamic evaluation is middle cerebral artery (MCA) flow (3) and pulsatility index (49). Reduced pulsatility index in the MCA demonstrates cerebral vasodilatation (and brain-sparing), and a number of studies show that MCA vasodilatation predicts neurodevelopmental deficits after birth (28, 50, 51, 52). All available data to date indicate that vasodilation of the MCA reflects an advanced and severe stage of growth restriction and brain injury, with high risk for abnormal neurodevelopment (53, 54). The anterior and posterior cerebral arteries might also provide cerebral hemodynamic insight, characteristic of the onset and the degree of brain-sparing (50). Scherjon and colleagues showed that fetal brain-sparing with elevated umbilical/cerebral ratio was associated with normal neurodevelopmental outcome at 3 years of age but, at 5 years, infants with brain-sparing had an IQ score 9 points lower than expected (30, 55). Fetal blood flow redistribution in favor of the fetal brain can also be detected and quantified by the Doppler cerebral/umbilical ratio (C/U ratio=cerebral resistance index (CRI)/umbilical resistance index) (56) and the fractional moving blood volume estimation (57). Fetal deterioration in chronic and severe hypoxia is characterized by the disappearance of physiological cerebral vascular variability, followed by an increase in cerebral vascular resistance (56). However, studies on growth-restricted and hypoxic human fetuses have shown that perinatal brain lesions can develop even before the loss of cerebrovascular variability (27). The cerebroplacental ratio (ratio of Doppler indices of MCA and UA) is an important predictor of adverse perinatal and later outcomes. The sensitivity and specificity of an abnormal cerebroplacental ratio for an adverse perinatal outcome lies between 60% and 80% (58).
Fetal ultrasound—others doppler studies
Aortic isthmus Doppler has been proposed as a novel method to interrogate oxygenation of the cerebral circulation in the presence of brain-sparing. When downstream placental vascular resistance is high and cerebral vascular resistance is low, blood leaving the right ventricle may take the path of least resistance and flow retrograde through the aortic isthmus. This results in poorly oxygenated blood from the right ventricle, destined for the placenta, instead being shunted through the aortic isthmus to the cerebral circulation (59). In the setting of early-onset severe FGR, retrograde flow increases the likelihood of IVH and periventricular leukomalacia (60). Not surprisingly, such retrograde flow has been linked with adverse neurodevelopmental outcomes at the age of 2–5 years (61).
UA Dopplers have also been studied, but their role in the assessment of brain injury or outcomes in FGR is not clear (62). Overall, fetal Dopplers greatly assist in the assessment and prediction of FGR, particularly the more severe cases, and, although these Doppler indices do not detect brain injury per se, they provide an essential first screening to identify fetuses at greatest risk of brain injury. Less severe FGR fetuses are more difficult to detect via Doppler assessments and, therefore, FGR and associated neuropathology may be missed.
Fetal ultrasound—direct assessment of brain structure
In addition to assessment of the fetoplacental circulation, ultrasound also offers opportunities to assess fetal brain structure. Prenatal 3D ultrasound can detect smaller brain volume in FGR fetuses (63), and can detect coexistent neuropathologies including intracranial hemorrhage and hydrocephalus, which may not be due to FGR but contribute to adverse outcomes (64). FGR fetuses show differences in the volume of many intracranial structures compared with appropriate for gestational age fetuses, with the largest difference observed in the frontal region. Nomograms exist for the ultrasonographic dimensions of the fetal corpus callosum, allowing for prenatal diagnosis of abnormal callosal development (65, 66). Cerebellar size, measured by ultrasound, is correlated with the severity of FGR, and therefore the trans-cerebellar diameter may also have prognostic significance (67). Although there are some studies that have correlated fetal corpus callosum and trans-cerebellar diameter changes with neurobehavior and neurodevelopment (68, 69, 70), these remain relatively rudimentary detecting only the most overt structural changes.
Fetal ultrasound—assessment of fetal behavior
Fetal biophysical profile, which assesses fetal tone, breathing, and body movements, has been traditionally used in the surveillance of high-risk pregnancies, and its accuracy in prediction of perinatal and neonatal outcomes continues to be debated (7). Kurjak et al. (71)proposed a scoring system for the assessment of fetal neurological status by 4D sonography named “Kurjak Antenatal Neurodevelopmental Test (KANET)”. This test assesses fetal behavior in a qualitative and quantitative manner (72); however, it remains unvalidated in large studies by independent operators and requires skill development and expertise that currently limits its widespread use.
In summary, a number of fetal ultrasound tools are available to directly and indirectly detect, assess, and prognosticate on neurological outcomes of FGR fetuses. Comprehensive evaluation of heterogenous FGR fetuses in large studies will aid elucidation of the most reliable and feasible neuroimaging assessments capable of predicting neurodevelopmental outcomes.
Fetal MRI
Fetal brain MRI has revolutionized early detection of intrauterine CNS injury in high-risk fetal and pregnancy conditions. Fetal MRI can be technically challenging, with acquisition of diagnostic quality fetal brain MRI affected by the trans-abdominal intrauterine environment and fetal movement. Fetal MRI brain is ideally done in a center with good radiological expertise, in late gestation when the fetal head is fixed in the maternal pelvis. In FGR, fetal brain MRI is currently used predominantly as a clinical tool to exclude gross brain malformations, and as a research tool for the evaluation of FGR-related brain injury; hence, predictive values for adverse outcomes are not yet available.
Fetal MRI—brain structure
Fetal MRI provides a sensitive and detailed assessment of the developing brain in high-risk conditions, including for growth-restricted fetuses, with the capacity to correlate fetal brain structural anomalies with neurodevelopmental outcomes (73). MRI of the fetus and the fetal brain has been used to confirm circulatory redistribution—brain-sparing—in FGR fetuses via assessment of fetal organ volumetry (13) or superior vena caval and umbilical vein perfusion (74). Fetal brain MRI has also been used in late-onset FGR fetuses to demonstrate an abnormal pattern of cortical development (75). Brain function, especially childhood development, is tightly linked to the development of the cortex in late gestation, and MRI with post-processing image analyses can provide insight into cortical development (76). This is reflected in the use of conventional MRI and diffusion tensor imaging (DTI) of appropriately grown and FGR fetuses, which has elucidated the relationship between changes in intracortical layering and cortical folding, where FGR is associated with altered cortical development (76). Fetal MRI of the corpus callosum has also confirmed that this structure is significantly smaller in FGR fetuses, and this is correlated with adverse neonatal neurobehavioral outcomes (68).
Fetal MRI—brain metabolism
Magnetic resonance spectroscopy (MRS) is a standard tool used in the early neonatal period to examine brain biochemistry wherein changes in MRS can predict neurological outcomes in perinatal brain injury, especially birth asphyxia (77). More recently, MRS has been successfully undertaken on the fetal brain, and to date demonstrates similar findings to those observed in neonatal populations with respect to altered brain metabolite concentrations within the compromised brain. MRS is particularly useful when subtle changes are present on conventional fetal MRI sequences (78). Brain-sparing in FGR fetuses is associated with altered brain metabolism evidenced by a reduction in the peak ratios of the metabolites N-acetylaspartate: Choline (NAA:Cho) and N-acetylaspartate: Creatine (NAA:Cr) (79). NAA is a very useful marker of neuronal cell integrity, and therefore reduction in these ratios principally reflects a loss of neurons within the FGR brain. Furthermore, frontal lobe NAA:Cho ratio in FGR and appropriately grown fetuses shows a strong association with corpus callosum development (80). The brain metabolite myo-inositol, considered a good marker of glial astrocyte cells, has also been examined in FGR and appropriately grown fetuses via MRS, but levels in key brain regions are not shown to be significantly different between cohorts (81).
In summary, a number of promising MRI and MRS tools for use in the fetus are currently being investigated. It is apparent that they can provide excellent direct assessments of structure and biochemistry of the developing brain, and to date results suggest that fetal MRI and MRS outcomes show strong predictive value for long-term neurodevelopmental outcomes. Fetal MRI is, however, not readily available across obstetric and birth centers, and requires specialist expertise, adequate training of radiology personnel involved, and resources to obtain reliable, clinically useful, and relevant information. Moving forward, it will be critical for individual centers to evaluate the pros and cons of obtaining fetal brain MRI assessments, with consideration for the expertise and resources available.
Detection of brain injury in the fgr neonate
In high-resource clinical settings, approximately half of the FGR fetuses are detected antenatally (82) (Figures 1 and 2). The findings presented above demonstrate that fetal assessment of the FGR brain could effectively be incorporated into routine clinical care to detect neuropathology associated with FGR, particularly in the most severe cases. However, in the remaining (antenatally undiagnosed) FGR neonates, it is critical that effective screening and detection strategies are in place for the assessment of neuropathology after birth. In the first instance, an important consideration is therefore the neonatal identification of the growth-restricted infant who would be appropriate for a newborn imaging examination. This allows for the possibility of neuroprotective strategies to treat FGR-related brain injury to commence shortly after birth, and certainly provides clinicians and parents with knowledge regarding diagnosis and follow-up requirements across the spectrum of potential outcomes.
Neonatal Ultrasound
Cranial ultrasound enables bedside, easily available serial cerebral assessment, and is commonly used as the primary brain-imaging modality in high-risk neonates, especially those born preterm. It is well described that significant brain abnormalities evident on neonatal cranial ultrasound are associated with adverse neurodevelopment (83). Neonatal cranial ultrasound is considered the gold-standard screening method for neonatal brain injury, for the detection of major or significant abnormalities of the brain, most notably severe IVH or cystic periventricular leukomalacia in the preterm infant. It is, however, not generally considered sensitive enough to detect and assess subtle or diffuse brain pathologies in the neonatal period (84), and most term-born growth-restricted infants are unlikely to have a brain lesion easily identifiable by neonatal cranial ultrasound. Overall, the sensitivity of neonatal ultrasound to detect any brain injury predictive of adverse motor outcomes at 2–3 years ranges between 20% and 60%, with specificity of 80–95% especially with severe injury, PPV of 20–60%, and NPV of 85–100% (85).
The interaction of prematurity and FGR on neonatal hemorrhagic and ischemic brain damage, as detected by cranial ultrasound, has been described and debated for over 20 years (86, 87). There is inconsistent evidence on whether placental insufficiency and FGR is directly linked to IVH and other neonatal cranial ultrasound abnormalities (88, 89, 90, 91). Some studies have shown FGR to be associated with an increased prevalence of IVH and white matter damage detectable on ultrasound brain scans in preterm neonates (92, 93, 94). In contrast, other studies have reported a reduced rate of IVH in FGR infants (95, 96) or have shown no change in the incidence of neonatal cranial ultrasound abnormalities in FGR infants compared with appropriately grown preterm infants (91, 97, 98). More recently, two studies have found an increase in cranial ultrasound abnormalities in preterm FGR infants compared with matched controls (40, 99). This spectrum of outcomes may exist because of different definitions of FGR, inclusion criteria, and quality of ultrasound technology used.
Neonatal MRI
MRI of the brain in the neonatal period is a gold standard for non-invasive structural assessment of the brain with excellent sensitivity and prognostic utility. Neonatal brain MRI is considered supplementary to routine and sequential cranial ultrasound, and is most commonly used in term and preterm infants with suspected brain injury or in infants considered high risk (for example, infants born extremely preterm). Advances in MRI technology and post-processing have greatly progressed our understanding of, and ability to detect, neonatal neuropathology, resulting in a broad shift from simply using neonatal MRI for the detection of severe cystic brain lesions toward the assessment of subtle and/or diffuse injury, or injury that is region-specific (84). MRI provides for a range of assessments of the neonatal brain that can be correlated with outcome measures of childhood motor and cognitive function, behavior, and learning (100). Specifically for motor outcomes, neonatal MRI demonstrates 80–100% sensitivity and specificity, PPV between 30 and 90%, and NPV of 90–100% (85).
Neonatal MRI—brain structure
More than a decade ago, Tolsa and colleagues used echo-planar MRI to demonstrate that FGR neonates imaged within 2 weeks after preterm birth demonstrate region-specific alterations in brain development, with decreased total brain volume and cortical (gray matter) volume compared with appropriately grown infants. A second MRI at term-equivalent age confirmed that the reduction in total intracranial and gray matter volume in FGR infants, with reduced cortical volume at term, correlated with worse behavioral outcomes (27). MRI examination of preterm-born FGR and appropriately grown infants at term-equivalent age found no differences in the incidence of gross brain lesions, or in the degree of morphological brain maturation between the two groups; however, there was a delay in myelination within the FGR cohort (101). Preterm FGR neonates have also been shown to have discordant gyrification and cortical folding observed on MRI soon after birth, which can predict term-equivalent-age cerebral volumes and neurobehavioral development (18). There are a number of studies that have shown structural differences (including in the gray matter) in the brain of FGR infant as compared with the appropriately grown infant at a later age (70, 102).
The hippocampus is a highly vulnerable brain structure that is altered in response to chronic fetal hypoxia and therefore frequently reported as abnormally developed in human and animal experimental FGR (2). The hippocampal structure of preterm-born FGR infants has been examined using 3D MRI at term-equivalent age, demonstrating reduced hippocampal gray matter volume. Hippocampal volume reduction was associated with functional behavioral differences at term-equivalent age, but not at 24 months of corrected age (103).
Neonatal MRI—brain metabolism
Although MRS is regularly used in the early assessment and diagnosis for acute neonatal encephalopathy associated with birth asphyxia (hypoxic ischemic encephalopathy), MRS has not been routinely used for assessment of the neonatal FGR brain. This is likely contributed by the difficulty in imaging FGR neonates very soon after birth, at a time when brain biochemical metabolites may be still be influenced by the chronic disturbances resultant from placental insufficiency. A recent study in neonatal FGR rabbits showed reduced NAA in the cerebral cortex and hippocampus, likely because of a loss of neuronal cells, and higher levels of glycine in the striatum. These metabolic changes were correlated with decreased brain volume (104). Similarly, regional differences in brain neurochemical profiles have been observed in FGR rats (105).
Neonatal MRI—brain organization and networks
A relatively new imaging tool allows the examination of brain organization using diffusion MRI, which we now appreciate, has the potential to describe complex brain connection networks, and to correlate these with neurodevelopmental outcomes. Specifically, MRI-based connectomics is an emerging approach to extract information from MRI data that exhaustively maps inter-regional connectivity within the brain to build a graph model of the neural circuitry forming the brain network (106, 107).
DTI assessment of fractional anisotropy (FA), which provides microstructural information on the density and organization of white matter tracts, provides an excellent assessment modality for white matter development within the brain. FGR is associated with a complex pattern of brain reorganization (as demonstrated by FA) in specific regions of the brain, as determined from voxel-based analysis in the neonatal period (107, 108). A recent study in FGR neonates showed hyper-connected but poorly organized brain networks that were most notable within the frontal, cingulate, and lingual cortices (109). A number of further brain connectivity studies have been performed at a later age (typically around 1 year of age or beyond) showing altered brain network organizations in infants born growth-restricted (22, 23, 102, 110, 111, 112). Importantly, two recent studies demonstrate that brain connectivity is predictive of subsequent functional delays in preterm and/or FGR infants (23, 113). The future study of neuropathology in FGR infants should incorporate examination of brain connectivity, which is emerging as a predictive assessment of complex brain reorganization that occurs in FGR infants in response to placental insufficiency.
In summary, advanced neonatal MRI of brain structure and microstructure, incorporating the use of DTI, is increasingly being used for the detection and assessment of FGR-related brain injury in infants after birth. MRI provides high resolution and therefore the capability for detecting subtle, but clinically important, brain-imaging information. It is becoming apparent that neonatal brain examination of FGR infants must incorporate detection of white matter injury and altered brain connectivity, and link with robust follow-up data. Altogether, these will provide evaluation on how brain microstructural changes correlate with long-term neurodevelopmental outcomes in FGR infants.
Other tools and techniques to detect fgr-related brain injury
A range of indirect assessment tools that have been used to identify the presence or severity of brain injury have been the subject of trials in growth-restricted fetuses and newborns. Visual evoked responses using magnetoencephalography provide a simple and non-invasive assessment of brain function, and are delayed in FGR fetuses (114). A number of other tools available in the neonatal period may predict long-term neurodevelopmental outcomes in FGR infants. In the first instance, this may be as simple as the measure of smaller head circumference in FGR infants, which is a good predictor for poor neurodevelopmental outcome (115). Although head circumference is an important predictor of neurodevelopmental outcome independent of gestational age, overall growth delay in the fetal period as impacted by the severity of FGR probably has the greatest impact in early-onset FGR (46). Overall, postnatal growth restriction, especially poor head growth, can have an additive impact on adverse neurodevelopment (116). Higher postnatal venous hematocrit and lower cerebral blood flow velocity have also been suggested as prognostic markers for adverse neurodevelopment in FGR neonates (117). Nuclear magnetic resonance spectroscopy-based analysis of umbilical vein blood in FGR infants has shown interesting patterns of metabolite change. Increased lipid levels were present in umbilical vein samples from both early and late FGR infants, whereas glucose was decreased and acetone increased in early FGR infants. FGR cases also showed increased glutamine and creatine levels, whereas the amounts of choline, valine, leucine, phenylalanine, and tyrosine were decreased in cord blood samples (118). S100B is a glial astrocyte protein that is released from brain astrocytes in response to injury, and elevated cord blood levels of S100B are associated with subsequent diagnosis of cerebral palsy (119).
Last, qualitative assessment of general movements (GMs) (120) is a powerful diagnostic method to evaluate brain dysfunction in “at-risk” preterm and term infants (121). Many infants with growth restriction have transient abnormal GMs in the early newborn period, indicating the importance of obtaining serial observations. As is the case with preterm infants, the quality of fidgety movements (when examined at 12 weeks post term age) is predictive for neuromotor outcome in term and preterm FGR infants (122, 123). Irrespective of imaging and other diagnostic modalities used, FGR infants should be closely followed up after birth to ascertain the impact of FGR on long-term neurodevelopmental outcomes, incorporating motor, cognition, and behavioral assessments, in this vulnerable population.
Conclusions
Detection and assessment of neuropathology in the fetus or neonate is a major challenge for modern perinatal medicine, allowing for timely delivery of the fetus, prediction of long-term consequences, and neuroprotective interventions. FGR is a common complication of pregnancy, and FGR infants have a greatly elevated risk for fetal and neonatal brain injury, such that strategies for the detection and treatment of FGR neuropathology are of great interest. We suggest that optimizing outcomes for FGR infants requires a collaborative approach, incorporating improved detection of true FGR infants during pregnancy via Doppler assessment of the degree of growth restriction. These infants in whom FGR is confirmed antenatally should provide a reference group for further validation studies incorporating biomarkers of brain injury, general movement assessment after birth, and direct assessment of the brain via cranial ultrasound and MRI in the neonatal period. For all FGR newborns, it is clear that early assessment of brain abnormalities should be a principal aim and, where possible, advanced MRI should be incorporated to provide clinicians and parents with accurate diagnostic information. There are currently no interventions or treatments that are available to improve brain development in FGR infants, and this should be a research focus to reduce the burden of neurodevelopmental impairments associated with FGR.
References
Ananth CV, Friedman AM . Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin Perinatol 2014;38:151–8.
Miller SL, Huppi PS, Mallard C . The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol 2016;594:807–23.
Levine TA, Grunau RE, McAuliffe FM, Pinnamaneni R, Foran A, Alderdice FA . Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics 2015;135:126–41.
Resnik R . Intrauterine growth restriction. Obstet Gynecol 2002;99:490–6.
Pollack RN, Divon MY . Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol 1992;35:99–107.
Gagnon R . Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol 2003;110 (Suppl 1):S99–S107.
Figueras F, Gardosi J . Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol 2011;204:288–300.
Soothill PW, Nicolaides KH, Campbell S . Prenatal asphyxia, hyperlacticaemia, hypoglycaemia, and erythroblastosis in growth retarded fetuses. Br Med J (Clin Res Ed) 1987;294:1051–3.
McMillen IC, Adams MB, Ross JT et al, Fetal growth restriction: adaptations and consequences. Reproduction 2001;122:195–204.
Cetin I, Alvino G . Intrauterine growth restriction: implications for placental metabolism and transport: a review. Placenta 2009;30:S77–S82.
Kamitomo M, Alonso JG, Okai T, Longo LD, Gilbert RD . Effects of long-term, high-altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol 1993;169:701–7.
Miller SL, Supramaniam VG, Jenkin G, Walker DW, Wallace EM . Cardiovascular responses to maternal betamethasone administration in the intrauterine growth-restricted ovine fetus. Am J Obstet Gynecol 2009;201:e611–8.
Damodaram MS, Story L, Eixarch E et al, Foetal volumetry using magnetic resonance imaging in intrauterine growth restriction. Early Hum Dev 2012;88:S35–S40.
Poudel R, McMillen IC, Dunn SL, Zhang S, Morrison JL . Impact of chronic hypoxemia on blood flow to the brain, heart, and adrenal gland in the late-gestation IUGR sheep fetus. Am J Physiol Regul Integr Comp Physiol 2015;308:R151–62.
Geva R, Eshel R, Leitner Y, Valevski AF, Harel S . Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics 2006;118:91–100.
Basilious A, Yager J, Fehlings MG . Neurological outcomes of animal models of uterine artery ligation and relevance to human intrauterine growth restriction: a systematic review. Dev Med Child Neurol 2015;57:420–30.
Samuelsen GB, Pakkenberg B, Bogdanovic N et al, Severe cell reduction in the future brain cortex in human growth-restricted fetuses and infants. Am J Obstet Gynecol 2007;197:56.e51–57.
Dubois J, Benders M, Borradori-Tolsa C et al, Primary cortical folding in the human newborn: an early marker of later functional development. Brain 2008;131:2028–41.
Tolcos M, Bateman E, O'Dowd R et al, Intrauterine growth restriction affects the maturation of myelin. Exp Neurol 2011;232:53–65.
Nitsos I, Rees S . The effects of intrauterine growth retardation on the development of neuroglia in fetal guinea pigs. An immunohistochemical and an ultrastructural study. Int J Dev Neurosci 1990;8:233–44.
Olivier P, Baud O, Bouslama M, Evrard P, Gressens P, Verney C . Moderate growth restriction: deleterious and protective effects on white matter damage. Neurobiol Dis 2007;26:253–63.
Fischi-Gomez E, Munoz-Moreno E, Vasung L et al, Brain network characterization of high-risk preterm-born school-age children. Neuroimage Clin 2016;11:195–209.
Eixarch E, Munoz-Moreno E, Bargallo N, Batalle D, Gratacos E . Motor and cortico-striatal-thalamic connectivity alterations in intrauterine growth restriction. Am J Obstet Gynecol 2016;214:725.e721–29.
Murray E, Fernandes M, Fazel M, Kennedy SH, Villar J, Stein A . Differential effect of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG 2015;122:1062–72.
Baschat AA . Neurodevelopment after fetal growth restriction. Fetal Diagn Ther 2014;36:136–42.
Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M . Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J Matern Fetal Neonatal Med 2013;26:222–5.
Tolsa CB, Zimine S, Warfield SK et al, Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res 2004;56:132–8.
Eixarch E, Meler E, Iraola A et al, Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol 2008;32:894–9.
Oros D, Figueras F, Cruz-Martinez R et al, Middle versus anterior cerebral artery Doppler for the prediction of perinatal outcome and neonatal neurobehavior in term small-for-gestational-age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 2010;35:456–61.
Scherjon S, Briet J, Oosting H, Kok J . The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic signs of fetal brain-sparing. Pediatrics 2000;105:385–91.
Walker DM, Marlow N . Neurocognitive outcome following fetal growth restriction. Arch Dis Child Fetal Neonatal Ed 2008;93:F322–5.
Schreuder AM, McDonnell M, Gaffney G, Johnson A, Hope PL . Outcome at school age following antenatal detection of absent or reversed end diastolic flow velocity in the umbilical artery. Arch Dis Child Fetal Neonatal Ed 2002;86:F108–14.
McIntyre S, Taitz D, Keogh J, Goldsmith S, Badawi N, Blair E . A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol 2013;55:499–508.
Blair EM, Nelson KB . Fetal growth restriction and risk of cerebral palsy in singletons born after at least 35 weeks' gestation. Am J Obstet Gynecol 2015;212:520.e521–7.
Malcus P . Antenatal fetal surveillance. Curr Opin Obstet Gynecol 2004;16:123–128.
Gordijn SJ, Beune IM, Thilaganathan B et al, Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016;48:333–9.
Unterscheider J, Daly S, Geary MP et al, Definition and management of fetal growth restriction: a survey of contemporary attitudes. Eur J Obstet Gynecol Reprod Biol 2014;174:41–5.
Figueras F, Gratacos E . Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther 2014;36:86–98.
Muresan D, Rotar IC, Stamatian F . The usefulness of fetal Doppler evaluation in early versus late onset intrauterine growth restriction. Review of the literature. Med Ultrason 2016;18:103–9.
Cruz-Martinez R, Tenorio V, Padilla N, Crispi F, Figueras F, Gratacos E . Risk of ultrasound-detected neonatal brain abnormalities in intrauterine growth-restricted fetuses born between 28 and 34 weeks' gestation: relationship with gestational age at birth and fetal Doppler parameters. Ultrasound Obstet Gynecol 2015;46:452–9.
Valcamonico A, Danti L, Frusca T et al, Absent end-diastolic velocity in umbilical artery: risk of neonatal morbidity and brain damage. Am J Obstet Gynecol 1994;170:796–801.
Marsoosi V, Bahadori F, Esfahani F, Ghasemi-Rad M . The role of Doppler indices in predicting intra ventricular hemorrhage and perinatal mortality in fetal growth restriction. Med Ultrason 2012;14:125–32.
Meyberg-Solomayer GC, Soen M, Speer R et al, Pathological prenatal Doppler sonography findings and their association with neonatal cranial ultrasound abnormalities in a high risk collective. Ultrasound Med Biol 2008;34:1193–9.
Vossbeck S, de Camargo OK, Grab D, Bode H, Pohlandt F . Neonatal and neurodevelopmental outcome in infants born before 30 weeks of gestation with absent or reversed end-diastolic flow velocities in the umbilical artery. Eur J Pediatr 2001;160:128–34.
Morsing E, Asard M, Ley D, Stjernqvist K, Marsal K . Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics 2011;127:e874–82.
Baschat AA . Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet Gynecol 2011;37:501–14.
Cosmi E, Ambrosini G, D'Antona D, Saccardi C, Mari G . Doppler, cardiotocography, and biophysical profile changes in growth-restricted fetuses. Obstet Gynecol 2005;106:1240–5.
Morris RK, Selman TJ, Verma M, Robson SC, Kleijnen J, Khan KS . Systematic review and meta-analysis of the test accuracy of ductus venosus Doppler to predict compromise of fetal/neonatal wellbeing in high risk pregnancies with placental insufficiency. Eur J Obstet Gynecol Reprod Biol 2010;152:3–12.
Aditya I, Tat V, Sawana A, Mohamed A, Tuffner R, Mondal T . Use of Doppler velocimetry in diagnosis and prognosis of intrauterine growth restriction (IUGR): a review. J Neonatal Perinatal Med 2016;9:117–26.
Hernandez-Andrade E, Serralde JA, Cruz-Martinez R . Can anomalies of fetal brain circulation be useful in the management of growth restricted fetuses? Prenat Diagn 2012;32:103–12.
Figueras F, Cruz-Martinez R, Sanz-Cortes M et al, Neurobehavioral outcomes in preterm, growth-restricted infants with and without prenatal advanced signs of brain-sparing. Ultrasound Obstet Gynecol 2011;38:288–94.
Spinillo A, Montanari L, Roccio M, Zanchi S, Tzialla C, Stronati M . Prognostic significance of the interaction between abnormal umbilical and middle cerebral artery Doppler velocimetry in pregnancies complicated by fetal growth restriction. Acta Obstet Gynecol Scand 2009;88:159–66.
Rossi A, Romanello I, Forzano L, Fachechi G, Marchesoni D . Evaluation of fetal cerebral blood flow perfusion using power Doppler ultrasound angiography (3D-PDA) in growth-restricted fetuses. Facts Views Vis Obgyn 2011;3:175–80.
Figueroa-Diesel H, Hernandez-Andrade E, Acosta-Rojas R, Cabero L, Gratacos E . Doppler changes in the main fetal brain arteries at different stages of hemodynamic adaptation in severe intrauterine growth restriction. Ultrasound Obstet Gynecol 2007;30:297–302.
Scherjon SA, Oosting H, Smolders-DeHaas H, Zondervan HA, Kok JH . Neurodevelopmental outcome at three years of age after fetal ‘brain-sparing’. Early Hum Dev 1998;52:67–79.
Salihagic-Kadic A, Medic M, Jugovic D et al, Fetal cerebrovascular response to chronic hypoxia—implications for the prevention of brain damage. J Matern Fetal Neonatal Med 2006;19:387–96.
Cruz-Martinez R, Figueras F, Hernandez-Andrade E, Benavides-Serralde A, Gratacos E . Normal reference ranges of fetal regional cerebral blood perfusion as measured by fractional moving blood volume. Ultrasound Obstet Gynecol 2011;37:196–201.
DeVore GR . The importance of the cerebroplacental ratio in the evaluation of fetal well-being in SGA and AGA fetuses. Am J Obstet Gynecol 2015;213:5–15.
Van Mieghem T, Hodges R, Jaeggi E, Ryan G . Functional echocardiography in the fetus with non-cardiac disease. Prenat Diagn 2014;34:23–32.
Fouron JC, Gosselin J, Raboisson MJ et al, The relationship between an aortic isthmus blood flow velocity index and the postnatal neurodevelopmental status of fetuses with placental circulatory insufficiency. Am J Obstet Gynecol 2005;192:497–503.
Cruz-Lemini M, Crispi F, Van Mieghem T et al, Risk of perinatal death in early-onset intrauterine growth restriction according to gestational age and cardiovascular Doppler indices: a multicenter study. Fetal Diagn Ther 2012;32:116–22.
Browne VA, Julian CG, Toledo-Jaldin L, Cioffi-Ragan D, Vargas E, Moore LG . Uterine artery blood flow, fetal hypoxia and fetal growth. Philos Trans R Soc Lond B Biol Sci 2015;370:20140068.
Businelli C, de Wit C, Visser GH, Pistorius LR . Ultrasound evaluation of cortical brain development in fetuses with intrauterine growth restriction. J Matern Fetal Neonatal Med 2015;28:1302–1307.
Kutuk MS, Yikilmaz A, Ozgun MT et al, Prenatal diagnosis and postnatal outcome of fetal intracranial hemorrhage. Childs Nerv Syst 2014;30:411–8.
Goldstein I, Tamir A, Reece AE, Weiner Z . Corpus callosum growth in normal and growth-restricted fetuses. Prenat Diagn 2011;31:1115–9.
Egana-Ugrinovic G, Savchev S, Bazan-Arcos C, Puerto B, Gratacos E, Sanz-Cortes M . Neurosonographic assessment of the corpus callosum as imaging biomarker of abnormal neurodevelopment in late-onset fetal growth restriction. Fetal Diagn Ther 2015;37:281–8.
Snijders RJ, De Courcy-Wheeler RH, Nicolaides KH . Intrauterine growth retardation and fetal transverse cerebellar diameter. Prenat Diagn 1994;14:1101–5.
Egana-Ugrinovic G, Sanz-Cortes M, Couve-Perez C, Figueras F, Gratacos E . Corpus callosum differences assessed by fetal MRI in late-onset intrauterine growth restriction and its association with neurobehavior. Prenat Diagn 2014;34:843–9.
Mallard C, Loeliger M, Copolov D, Rees S . Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following intrauterine growth-restriction. Neuroscience 2000;100:327–33.
Padilla N, Falcon C, Sanz-Cortes M et al, Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: a magnetic resonance imaging study. Brain Res 2011;1382:98–108.
Kurjak A, Predojevic M, Stanojevic M et al, Intrauterine growth restriction and cerebral palsy. Acta Inform Med 2012;18:64–82.
Kurjak A, Talic A, Honemeyer U, Stanojevic M, Zalud I . Comparison between antenatal neurodevelopmental test and fetal Doppler in the assessment of fetal well being. J Perinat Med 2013;41:107–14.
Banovic V, Skrablin S, Banovic M, Rados M, Gveric-Ahmetasevic S, Babic I . Fetal brain magnetic resonance imaging and long-term neurodevelopmental impairment. Int J Gynaecol Obstet 2014;125:237–40.
Zhu MY, Milligan N, Keating S et al, The hemodynamics of late onset intrauterine growth restriction by MRI. Am J Obstet Gynecol 2015;214:367.e1–e17.
Egana-Ugrinovic G, Sanz-Cortes M, Figueras F, Bargallo N, Gratacos E . Differences in cortical development assessed by fetal MRI in late-onset intrauterine growth restriction. Am J Obstet Gynecol 2013;209:126.e121–28.
Huppi PS . Cortical development in the fetus and the newborn: advanced MR techniques. Top Magn Reson Imag 2011;22:33–8.
Barkovich AJ, Baranski K, Vigneron D et al, Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol 1999;20:1399–405.
Pugash D, Krssak M, Kulemann V, Prayer D . Magnetic resonance spectroscopy of the fetal brain. Prenat Diagn 2009;29:434–41.
Story L, Damodaram MS, Allsop JM et al, Brain metabolism in fetal intrauterine growth restriction: a proton magnetic resonance spectroscopy study. Am J Obstet Gynecol 2011;205:483.e481–8.
Sanz-Cortes M, Egana-Ugrinovic G, Simoes RV, Vazquez L, Bargallo N, Gratacos E . Association of brain metabolism with sulcation and corpus callosum development assessed by MRI in late-onset small fetuses. Am J Obstet Gynecol 2015;212:804.e801–8.
Story L, Damodaram MS, Supramaniam V et al, Myo-inositol metabolism in appropriately grown and growth-restricted fetuses: a proton magnetic resonance spectroscopy study. Eur J Obstet Gynecol Reprod Biol 2013;170:77–81.
Sovio U, White IR, Dacey A, Pasupathy D, Smith GC . Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the pregnancy outcome prediction (POP) study: a prospective cohort study. Lancet 2015;386:2089–97.
Wezel-Meijler G, de Vries LS . Cranial ultrasound - optimizing utility in the NICU. Curr Pediatr Rev 2014;10:16–27.
de Vries LS, Benders MJ, Groenendaal F . Progress in neonatal neurology with a focus on neuroimaging in the preterm infant. Neuropediatrics 2015;46:234–41.
de Vries LS, Benders MJNL, Groenendaal F . Imaging the premature brain: ultrasound or MRI? Neuroradiology 2013;55:13–22.
Amato M, Konrad D, Huppi P, Donati F . Impact of prematurity and intrauterine growth retardation on neonatal hemorrhagic and ischemic brain damage. Eur Neurol 1993;33:299–303.
Damodaram M, Story L, Kulinskaya E, Rutherford M, Kumar S . Early adverse perinatal complications in preterm growth-restricted fetuses. Aust N Z J Obstet Gynaecol 2011;51:204–9.
Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A . Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol 2000;182:198–206.
Gilbert WM, Danielsen B . Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol 2003;188:1596–9.
von Beckerath AK, Kollmann M, Rotky-Fast C, Karpf E, Lang U, Klaritsch P . Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol 2013;208:130.e131–6.
Malhotra A, Yahya Z, Sasi A et al, Does fetal growth restriction lead to increased brain injury as detected by neonatal cranial ultrasound in premature infants? J Paediatr Child Health 2015;51:1103–8.
Padilla-Gomes NF, Enriquez G, Acosta-Rojas R, Perapoch J, Hernandez-Andrade E, Gratacos E . Prevalence of neonatal ultrasound brain lesions in premature infants with and without intrauterine growth restriction. Acta Paediatr 2007;96:1582–7.
McElrath TF, Allred EN, Boggess KA et al, Maternal antenatal complications and the risk of neonatal cerebral white matter damage and later cerebral palsy in children born at an extremely low gestational age. Am J Epidemiol 2009;170:819–28.
Baschat AA, Gembruch U, Viscardi RM, Gortner L, Harman CR . Antenatal prediction of intraventricular hemorrhage in fetal growth restriction: what is the role of Doppler? Ultrasound Obstet Gynecol 2002;19:334–9.
Aucott SW, Donohue PK, Northington FJ . Increased morbidity in severe early intrauterine growth restriction. J Perinatol 2004;24:435–40.
Mari G, Abuhamad AZ, Keller M, Verpairojkit B, Ment L, Copel JA . Is the fetal brain-sparing effect a risk factor for the development of intraventricular hemorrhage in the preterm infant? Ultrasound Obstet Gynecol 1996;8:329–32.
Simchen MJ, Beiner ME, Strauss-Liviathan N et al, Neonatal outcome in growth-restricted versus appropriately grown preterm infants. Am J Perinatol 2000;17:187–92.
Claris O, Besnier S, Lapillonne A, Picaud JC, Salle BL . Incidence of ischemic-hemorrhagic cerebral lesions in premature infants of gestational age <or =28 weeks: a prospective ultrasound study. Biol Neonate. 1996;70:29–34.
Starcevic M, Predojevic M, Butorac D, Tumbri J, Konjevoda P, Kadic AS . Early functional and morphological brain disturbances in late-onset intrauterine growth restriction. Early Hum Dev 2016;93:33–8.
Sizonenko SV, Borradori-Tolsa C, Bauthay DM, Lodygensky G, Lazeyras F, Huppi P . Impact of intrauterine growth restriction and glucocorticoids on brain development: insights using advanced magnetic resonance imaging. Mol Cell Endocrinol 2006;254-255:163–71.
Ramenghi LA, Martinelli A, De Carli A et al, Cerebral maturation in IUGR and appropriate for gestational age preterm babies. Reprod Sci 2011;18:469–75.
Padilla N, Junque C, Figueras F et al, Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res 2014;1545:1–11.
Lodygensky GA, Seghier ML, Warfield SK et al, Intrauterine growth restriction affects the preterm infant's hippocampus. Pediatr Res 2008;63:438–43.
Simoes RV, Munoz-Moreno E, Carbajo RJ et al, In vivo detection of perinatal brain metabolite changes in a rabbit model of intrauterine growth restriction (IUGR). PLoS ONE 2015;10:e0131310.
Maliszewski-Hall AM, Alexander M, Tkac I, Oz G, Rao R . Differential effects of intrauterine growth restriction on the regional neurochemical profile of the developing rat brain. Neurochem Res. 2015;42:133–40.
Wang J, Zuo X, He Y . Graph-based network analysis of resting-state functional MRI. Front Syst Neurosci 2010;4:16.
van de Looij Y, Dean JM, Gunn AJ, Huppi PS, Sizonenko SV . Advanced magnetic resonance spectroscopy and imaging techniques applied to brain development and animal models of perinatal injury. Int J Dev Neurosci 2015;45:29–38.
Eixarch E, Batalle D, Illa M et al, Neonatal neurobehavior and diffusion MRI changes in brain reorganization due to intrauterine growth restriction in a rabbit model. PLoS ONE 2012;7:e31497.
Batalle D, Munoz-Moreno E, Tornador C et al, Altered resting-state whole-brain functional networks of neonates with intrauterine growth restriction. Cortex 2016;77:119–31.
Illa M, Eixarch E, Batalle D et al, Long-term functional outcomes and correlation with regional brain connectivity by MRI diffusion tractography metrics in a near-term rabbit model of intrauterine growth restriction. PLoS ONE 2013;8:e76453.
Batalle D, Munoz-Moreno E, Arbat-Plana A et al, Long-term reorganization of structural brain networks in a rabbit model of intrauterine growth restriction. Neuroimage 2014;100:24–38.
Batalle D, Eixarch E, Figueras F et al, Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage 2012;60:1352–66.
Ball G, Pazderova L, Chew A et al, Thalamocortical connectivity predicts cognition in children born preterm. Cereb Cortex. 2015;25:4310–8.
Morin EC, Schleger F, Preissl H et al, Functional brain development in growth-restricted and constitutionally small fetuses: a fetal magnetoencephalography case–control study. BJOG 2015;122:1184–90.
Gale CR, O'Callaghan FJ, Bredow M, Martyn CN . The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 2006;118:1486–92.
Fattal-Valevski A, Toledano-Alhadef H, Leitner Y, Geva R, Eshel R, Harel S . Growth patterns in children with intrauterine growth retardation and their correlation to neurocognitive development. J Child Neurol 2009;24:846–51.
Basu S, Dewangan S, Barman S, Shukla RC, Kumar A . Postnatal changes in cerebral blood flow velocity in term intra-uterine growth-restricted neonates. Paediatr Int Child Health 2014;34:189–93.
Sanz-Cortes M, Carbajo RJ, Crispi F, Figueras F, Pineda-Lucena A, Gratacos E . Metabolomic profile of umbilical cord blood plasma from early and late intrauterine growth restricted (IUGR) neonates with and without signs of brain vasodilation. PLoS ONE 2013;8:e80121.
Costantine MM, Weiner SJ, Rouse DJ et al, Umbilical cord blood biomarkers of neurologic injury and the risk of cerebral palsy or infant death. Int J Dev Neurosci 2011;29:917–22.
Hagmann CF, De Vita E, Bainbridge A et al, T2 at MR imaging is an objective quantitative measure of cerebral white matter signal intensity abnormality in preterm infants at term-equivalent age. Radiology 2009;252:209–17.
Bosanquet M, Copeland L, Ware R, Boyd R . A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol 2013;55:418–26.
Bos AF, Einspieler C, Prechtl HF . Intrauterine growth retardation, general movements, and neurodevelopmental outcome: a review. Dev Med Child Neurol 2001;43:61–8.
Zuk L, Harel S, Leitner Y, Fattal-Valevski A . Neonatal general movements: an early predictor for neurodevelopmental outcome in infants with intrauterine growth retardation. J Child Neurol 2004;19:14–8.
Acknowledgements
AM is supported by a Royal Australasian College of Physicians Foundation Research Scholarship; GRP is supported by a National Health and Medical Research Council Fellowship; and SM is supported by an Australian Research Council Future Fellowship. We would also wish to acknowledge the Victorian Government’s Operational Infrastructure Support program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Malhotra, A., Ditchfield, M., Fahey, M. et al. Detection and assessment of brain injury in the growth-restricted fetus and neonate. Pediatr Res 82, 184–193 (2017). https://doi.org/10.1038/pr.2017.37
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.37
This article is cited by
-
Metabolic dynamics and prediction of sFGR and adverse fetal outcomes: a prospective longitudinal cohort study
BMC Medicine (2023)
-
miR-181d-5p, which is upregulated in fetal growth restriction placentas, inhibits trophoblast fusion via CREBRF
Journal of Assisted Reproduction and Genetics (2023)
-
Electroencephalographic studies in growth-restricted and small-for-gestational-age neonates
Pediatric Research (2022)
-
Fetal ischemia monitoring with in vivo implanted electrochemical multiparametric microsensors
Journal of Biological Engineering (2021)
-
Perinatal blood biomarkers for the identification of brain injury in very low birth weight growth-restricted infants
Journal of Perinatology (2021)