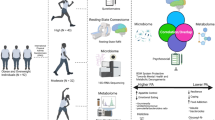
Abstract
Objective
The amygdala is importantly involved in stress and obesity, but its role on weight change and diet-related stress remains unexplored among adolescents with excess weight. We aimed to examine the functional connectivity of the Central and Basolateral amygdala nuclei (CeA and BLA) among adolescents, and to explore the longitudinal association between brain connectivity measures and diet-related cortisol and weight loss in adolescents with excess weight.
Methods
We compared resting-state functional connectivity between adolescents with excess (EW, N = 34; Age = 16.44 ± 1.66) and normal weight (NW, N = 36; Age = 16.50 ± 1.40) using a seed-based (CeA and BLA) whole-brain approach. Then, in a subset of 30 adolescents with EW, followed-up after 3-months of dietary/lifestyle intervention, we explored for interactions between connectivity in the CeA/BLA networks and weight loss. Regression analyses were performed to explore the relationship between accumulated cortisol and weight loss, and to test the potential effect of the amygdala networks on such association.
Results
In EW compared with NW, the CeA regions showed higher functional connectivity with anterior portions, and lower connectivity with posterior portions of the cingulate cortex, while the left BLA regions showed lower connectivity with the dorsal caudate and angular gyrus. In addition, higher connectivity between the left CeA-midbrain network was negatively associated with weight loss. Hair cortisol significantly predicted weight change (p = 0.012). However, this association was no longer significant (p = 0.164) when considering the CeA-midbrain network in the model as an additional predictor.
Conclusions
Adolescents with EW showed functional connectivity alterations within the BLA/CeA networks. The CeA-midbrain network might constitute an important brain pathway regulating weight change.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
NCD-Risc: Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–42.
Dockray S, Susman EJ, Dorn LD. Depression, cortisol reactivity and obesity in childhood and adolescence. J Adolesc Health. 2009;45:344–50.
Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–58.
Martín-Pérez C, Contreras-Rodríguez O, Vilar-López R, Verdejo-García A. Hypothalamic networks in adolescents with excess weight: stress-related connectivity and associations with emotional eating. J Am Acad Child Adolesc Psychiatry. 2019; 58:211–220.e5.
Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, Araujo IE, Gitelman DR, et al. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci. 2015;35:7964–76.
Tomiyama AJ, Mann T, Vinas D, Hunger JM, DeJager J, Taylor SE. Low calorie dieting increases cortisol. Psychosom Med. 2011;72:357–64.
Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav. 2007;91:432–9.
Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. Journal of Neuroscience. 2010;30:16399–407.
Paolini BM, Laurienti PJ, Simpson SL, Burdette JH, Lyday RG, Rejeski WJ. Global integration of the hot-state brain network of appetite predicts short term weight loss in older adult. Front Aging Neurosci. 2015;7. https://doi.org/10.3389/fnagi.2015.00070.
Hermann P, Gál V, Kóbor I, Kirwan B, Kovács P, Kitka T, et al. Efficacy of weight loss intervention can be predicted based on early alterations of fMRI food cue reactivity in the striatum. NeuroImage: Clin. 2019;101803. https://doi.org/10.1016/j.nicl.2019.101803.
Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2008;198:149–58.
Zhang Q, Li H, Guo F.Amygdala, an important regulator for food intake. Front Biol.2011;6:82–5.
Veer IM, Oei NYL, Spinhoven P, van Buchen MA, Elzinga BM, Rombouts SARB. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2012;37:1039–47.
Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cognit Sci. 2010;15:85–93.
Van Marle HJF, Hermans EJ, Qin S, Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. NeuroImage. 2010;53:348–54.
Taren AA, Gianaros PJ, Greco CM, Lindsay EK, Fairgrieve A, Brown K, et al. Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: a randomized controlled trial. Soc Cognitive Affect Neurosci. 2015;10:1758–68.
Horstmann A. The brain’s got a taste for good food. In: Avena NM editor. Hedonic eating: how the pleasure of food affects our brains and behavior. Oxford University Press: New York, USA; 2015. p. 39–56.
Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring H-U, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2011;33:1052–61.
Lips MA, Wijngaarden MA, Van der Grond J, Van Buchem MA, De Groot GH, Rombouts SA, et al. Resting-state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. Am J Clin Nutr. 2014;100:524–31.
Kahathuduwa CN, Davis T, O’Boyle M, Boyd LA, Chin S-H, Paniukov D, et al. Effects of 3-week total meal replacement vs. typical food-based diet on human brain functional magnetic resonance imaging food-cue reactivity and functional connectivity in people with obesity. Appetite. 2018;120:431–41.
Fowler CH, Miernicki ME, Rudolph KD, Telzer EH. Disrupted amygdala-prefrontal connectivity during emotion regulation links stress-reactive rumination and adolescent depressive symptoms. Dev Cogn Neurosci. 2017;27:99–106.
Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–41.
Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2013;34:3247–66.
Campbell-Smith EJ, Holmes NM, Lingawi NW, Panayi MC, Westbrook RF. Oxytocin signaling in basolateral and central amygdala nuclei differentially regulates the acquisition, expression, and extinction of context-conditioned fear in rats. Learn Mem. 2015;22:247–57.
Zseli G, Vida B, Szilvásy-Szabó A, Tóth M, Lechan RM, Fekete C. Neuronal connections of the central amygdalar nucleus with refeeding-activated brain areas in rats. Brain Struct Funct. 2018;223:391–414.
Roozendaal B, Koolhaas JM, Bohus B. The role of the central amygdala in stress and adaption. Acta Physiol Scand, Suppl. 1997;640:51–54.
Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron. 2017;93:1464–79.
Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA. 2003;100:11696–701.
Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci.2005;25:8295–302.
Petrovich GA, Ross CA, Mody P, Holland PC, Gallagher M. Central, but not basolateral, amygdala is critical for control of feeding by aversive learned cues. J Neurosci. 2009;29:15205–12.
Mietus-Snyder ML, Lustig RH. Childhood obesity: adrift in the “limbic triangle”. Annu Rev Med. 2008;59:147–62.
Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–65.
Tryon MS, Carter CS, DeCant R, Laugero KD. Chronic stress exposure may affect the brain´s response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav. 2013;120:233–42.
Sixty-fourth world health assembly. Resolution WHA 64.28: Youth and health risks. Geneva, World Health Organization; 2011.
Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. National Health Stat Report. 2010;25:1–5.
Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601.
Vanaelst B, Huybrechts I, Bammann K, Michels N, Vriendt T, et al. Intercorrelations between serum, salivary, and hair cortisol and child‐reported estimates of stress in elementary school girls. Psychophysiology. 2012;49:1072–81.
Serra-Majem LL, Aranceta J, Ribas L, Sangil M, Pérez C. El cribado de riesgo nutricional en Pediatría. Validación del test rápido KRECE PLUS y resultados en la población española. In: Serra LL, Aranceta J editors. Crecimiento y desarrollo: Dimensión alimentaria y nutricional. Masson: Barcelona, España; 2013, p. 45–55.
Whitefield-Gabrielli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54.
Brett M, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Neuroimage. 2003;16 (Supplement 1).
Baur V, Hänggi J, Langer N, Jäncke L. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;1:85–92.
Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031.
Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, et al. Beyond amygdala: default mode network activity differs between patients with social phobia and healthy controls. Brain Res Bull. 2009;79:409–13.
Veer IM, Oei NYL, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SARB. Beyond acute social stress: Increased functional connectivity between amygdala and cortical midline structures. NeuroImage. 2011;57:1534–41.
Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S. Elevated body mass index is associated with increased integration and reduced cohesion of sensory-driven and internally guided resting-state functional brain networks. Cerebr Cortex. 2017;28:988–97.
Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–34.
Kim J, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–10.
Kujawa A, Wu M, Klumpp H, Pine DS, Swain JE, Fitzgerald KD, et al. Altered development of amygdala-anterior cingulate cortex connectivity in anxious youth and young adults. Biol Psychiatry Cogn Neurosci Neuroimag. 2016;1:345–52.
Kubiak T, Vogele C, Siering M, Schiel R, Weber H. Daily hassles and emotional eating in obese adolescents under restricted dietary conditions-the role of ruminative thinking. Appetite. 2008;51:206–9.
Gearhardt AN, Yokum S, Orr PT. (2011). Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68:808.
Musgrove DR, Eberly LE, Klimes-Dougan B, Basgoze Z, Thomas KM, Mueller BA, et al. Impaired bottom-up effective connectivity between amygdala and subgenual anterior cingulate cortex in unmedicated adolescents with major depression: results from a dynamic causal modeling analysis. Brain Connect. 2015;5:608–19.
Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, et al. Dorsal and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One. 2012;7:e31089.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35.
Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–9.
Jackson SE, Kirschbaum C, Steptoe A. Hair cortisol and adiposity in a population-based sample of 2527 men and women aged 54 to 87 years. Obesity. 2017;25:539–44.
Acknowledgements
This study has been funded by the Project NEUROECOBE (HUM-6635), granted by the Andalusian Council of Innovation, Science and Industry, Spain. OCR is funded by Postdoctoral “PERIS” Contract (SLT006/17/00236) from the Health Department of the Catalan Government, Spain. JVR is supported by a grant from the Spanish Ministry of Science, Innovation and Universities (FJCI-2017-33396). AVG was funded by grants MRF1141214 from the Australian Medical Research Future Fund and GNT1140197 from the National Health and Medical Research Council.
Disclosure
All authors report no biomedical financial interests or any potential competing interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Martín-Pérez, C., Contreras-Rodríguez, O., Verdejo-Román, J. et al. Stressing diets? Amygdala networks, cumulative cortisol, and weight loss in adolescents with excess weight. Int J Obes 44, 2001–2010 (2020). https://doi.org/10.1038/s41366-020-0633-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-0633-4