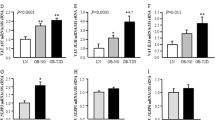
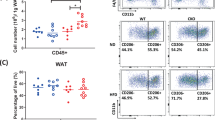
Abstract
Background/aims
Inflammation governs adipose tissue (AT) dysfunction in obesity. Retinoic acid receptor-related orphan receptor alpha (RORα) is associated with inflammation and insulin resistance in animal studies, but its role in human obesity remains elusive. We investigated the expression and function of RORα on AT inflammation in patients with morbid obesity with/without diabetes.
Subjects/methods
We assessed RORα expression in paired biopsies of subcutaneous and omental AT from 41 patients (body mass index (BMI) 43.3 ± 0.8 kg/m2) during Roux-en-Y-gastric surgery and explored the functional consequences of pharmacological RORα blockade in AT ex vivo.
Results
RORα expression was significantly higher in omental AT than in subcutaneous AT (p = 0.03) and was positively associated with BMI (r = 0.344, p = 0.027) and homeostasis model assessment of insulin resistance (r = 0.319, p = 0.041). In ex vivo assays, IL-8/CXCL8 and MCP-1/CCL2 chemokine release was significantly higher in omental fat explants from diabetic patients than from non-diabetics and was significantly diminished by RORα blockade (p < 0.05). Inhibition of RORα improved protein kinase B signaling and decreased NF-κB activity in omental AT from patients with diabetes (p < 0.05). Under dynamic flow conditions, RORα blockade prevented mononuclear cell attachment to human dysfunctional endothelial cells.
Conclusions
RORα blockade represents a potential therapy to prevent AT dysfunction and inflammation associated with insulin resistance in human obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–71.
Booth A, Magnuson A, Foster M. Detrimental and protective fat: body fat distribution and its relation to metabolic disease. Horm Mol Biol Clin Investig. 2014;17:13–27.
Mathieu P, Boulanger MC, Despres JP. Ectopic visceral fat: a clinical and molecular perspective on the cardiometabolic risk. Rev Endocr Metab Disord. 2014;15:289–98.
Hueso L, Ortega R, Selles F, Wu-Xiong NY, Ortega J, Civera M, et al. Upregulation of angiostatic chemokines IP-10/CXCL10 and I-TAC/CXCL11 in human obesity and their implication for adipose tissue angiogenesis. Int J Obes. 2018;42:1406–17.
Collado A, Marques P, Escudero P, Rius C, Domingo E, Martinez-Hervás S, et al. Functional role of endothelial CXCL16/CXCR6-platelet-leucocyte axis in angiotensin II-associated metabolic disorders. Cardiovasc Res. 2018;114:1764–75.
Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118:1786–807.
Zuriaga MA, Fuster JJ, Gokce N, Walsh K. Humans and mice display opposing patterns of “browning” gene expression in visceral and subcutaneous white adipose tissue depots. Front Cardiovasc Med. 2017;4:27.
Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–20.
Weikum ER, Liu X, Ortlund EA. The nuclear receptor superfamily: a structural perspective. Protein Sci. 2018;27:1876–92.
Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003.
Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, et al. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature. 1996;379:736–9.
Steinmayr M, Andre E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, Auclair N, et al. staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice. Proc Natl Acad Sci USA. 1998;95:3960–5.
Hirose T, Smith RJ, Jetten AM. ROR gamma: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem Biophys Res Commun. 1994;205:1976–83.
Besnard S, Heymes C, Merval R, Rodriguez M, Galizzi JP, Boutin JA, et al. Expression and regulation of the nuclear receptor RORalpha in human vascular cells. FEBS Lett. 2002;511:36–40.
Kang HS, Okamoto K, Takeda Y, Beak JY, Gerrish K, Bortner CD, et al. Transcriptional profiling reveals a role for RORalpha in regulating gene expression in obesity-associated inflammation and hepatic steatosis. Physiol Genom. 2011;43:818–28.
Billon C, Sitaula S, Burris TP. Inhibition of RORalpha/gamma suppresses atherosclerosis via inhibition of both cholesterol absorption and inflammation. Mol Metab. 2016;5:997–1005.
Pedro T, Martinez-Hervas S, Tormo C, Garcia-Garcia AB, Saez-Tormo G, Ascaso JF, et al. Oxidative stress and antioxidant enzyme values in lymphomonocytes after an oral unsaturated fat load test in familial hypercholesterolemic subjects. Transl Res. 2013;161:50–56.
Vitseva OI, Tanriverdi K, Tchkonia TT, Kirkland JL, McDonnell ME, Apovian CM, et al. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity. 2008;16:932–7.
Rice DR, White AG, Leevy WM, Smith BD. Fluorescence imaging of interscapular brown adipose tissue in living mice. J Mater Chem B. 2015;3:1979–89.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Ortega R, Collado A, Selles F, Gonzalez-Navarro H, Sanz MJ, Real JT, et al. SGLT-2 (sodium-glucose cotransporter 2) inhibition reduces ang II (angiotensin II)-induced dissecting abdominal aortic aneurysm in ApoE (apolipoprotein E) knockout mice. Arterioscler Thromb Vasc Biol. 2019;39:1614–28.
Furio E, García-Fuster MJ, Redon J, Marques P, Ortega R, Sanz MJ, et al. CX3CR1/CX3CL1 axis mediates platelet-leukocyte adhesion to arterial endothelium in younger patients with a history of idiopathic deep vein thrombosis. Thromb Haemost. 2018;118:562–71.
Cignarelli A, Genchi VA, Perrini S, Natalicchio A, Laviola L, Giorgino F. Insulin and insulin receptors in adipose tissue development. Int J Mol Sci. 2019;20:759–79.
Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, et al. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009;4:215–22.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Investig. 2006;116:1793–801.
Catalan V, Gomez-Ambrosi J, Ramirez B, Rotellar F, Pastor C, Silva C, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–74.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91.
Cartier A, Cote M, Bergeron J, Almeras N, Tremblay A, Lemieux I, et al. Plasma soluble tumour necrosis factor-alpha receptor 2 is elevated in obesity: specific contribution of visceral adiposity. Clin Endocrinol. 2010;72:349–57.
Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes. 2009;33:54–66.
Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76.
Liu Y, Chen Y, Zhang J, Liu Y, Zhang Y, Su Z. Retinoic acid receptor-related orphan receptor alpha stimulates adipose tissue inflammation by modulating endoplasmic reticulum stress. J Biol Chem. 2017;292:13959–69.
Lau P, Fitzsimmons RL, Pearen MA, Watt MJ, Muscat GE. Homozygous staggerer (sg/sg) mice display improved insulin sensitivity and enhanced glucose uptake in skeletal muscle. Diabetologia. 2011;54:1169–80.
Auclair M, Roblot N, Capel E, Fève B, Antoine B. Pharmacological modulation of RORα controls the fat browning, adaptive thermogenesis and body weight in mice. Am J Physiol Endocrinol Metab. 2021;320:E219–E33.
Monnier C, Auclair M, Le Cam G, Garcia MP, Antoine B. The nuclear retinoid-related orphan receptor RORα controls circadian thermogenic programming in white fat depots. Physiol Rep. 2018;6:e13678.
Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Investig. 2006;116:1494–505.
Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Investig. 2006;116:115–24.
Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93:3215–21.
Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 2008;28:1897–908.
Kumar N, Kojetin DJ, Solt LA, Kumar KG, Nuhant P, Duckett DR, et al. Identification of SR3335 (ML-176): a synthetic RORalpha selective inverse agonist. ACS Chem Biol. 2011;6:218–22.
Solt LA, Banerjee S, Campbell S, Kamenecka TM, Burris TP. ROR inverse agonist suppresses insulitis and prevents hyperglycemia in a mouse model of type 1 diabetes. Endocrinology. 2015;156:869–81.
Barbarroja N, López-Pedrera R, Mayas MD, García-Fuentes E, Garrido-Sánchez L, Macías-González M, et al. The obese healthy paradox: is inflammation the answer? Biochem J. 2010;430:141–9.
Kadiri S, Auclair M, Capeau J, Antoine B. Depot-specific response of adipose tissue to diet-induced inflammation: the retinoid-related orphan receptor alpha (RORalpha) involved? Obesity. 2017;25:1948–55.
Lau P, Fitzsimmons RL, Raichur S, Wang SC, Lechtken A, Muscat GE. The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J Biol Chem. 2008;283:18411–21.
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8.
Tourniaire F, Romier-Crouzet B, Lee JH, Marcotorchino J, Gouranton E, Salles J, et al. Chemokine expression in inflamed adipose tissue is mainly mediated By NF-κB. PLoS ONE. 2013;8:e66515.
Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, et al. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23:5651–63.
Engin A. Endothelial dysfunction in obesity. Adv Exp Med Biol. 2017;960:345–79.
Marques P, Collado A, Martinez-Hervás S, Domingo E, Benito E, Piqueras L, et al. Systemic Inflammation in MetabolicSyndrome: Increased Platelet and Leukocyte Activation, and Key Role of CX3CL1/CX3CR1 and CCL2/CCR2 Axes in Arterial Platelet-Proinflammatory Monocyte Adhesion. J Clin Med. 2019;8:708.
Weber KS, von Hundelshausen P, Clark-Lewis I, Weber PC, Weber C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur J Immunol. 1999;29:700–12.
Acknowledgements
This work was supported by grants from the Carlos III Health Institute/ Spanish Ministry of Health (PI18/00209, PI15/00082) and the Spanish Ministry of Economy and Competitiveness (SAF2017-89714-R), Generalitat Valenciana (grants Gent T CDEI-04/20-A and AICO/2019/250) and the European Regional Development Fund (FEDER). Hueso L holds a predoctoral grant from Carlos III Health Institute (FI19/00033). Piqueras L is under a “Plan GenT” contract (CDEI-04/20-A) (Conselleria de Sanidad, Valencia, Spain). The authors gratefully acknowledge the valuable collaboration of all the members of the multidisciplinary research Team of CIBERDEM: Diabetes and Associated Metabolic Diseases Networking Biomedical Research.
Author information
Authors and Affiliations
Contributions
Data curation, RO, and LH; formal analysis, RO, LH, MC, MJS, LP, and JTR; funding acquisition, LP, MJS, and JTR; investigation, RO, LH; methodology, RO, LH, EB, MC, JO, MJS, JTR, and LP; supervision, LP, MJS, and JTR; conceptualization and writing-original draft, LP; writing-review & editing, RO, LH, EB, MC, JO, MJS, JTR, and LP. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ortega, R., Hueso, L., Benito, E. et al. The nuclear retinoid‐related orphan receptor RORα controls adipose tissue inflammation in patients with morbid obesity and diabetes. Int J Obes 45, 1369–1381 (2021). https://doi.org/10.1038/s41366-021-00787-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00787-5
This article is cited by
-
Association Between RORA Polymorphisms and Obesity
Biochemical Genetics (2024)