Abstract
Neonatal drug and device development has lagged behind other patient populations. Oftentimes, providers are using drugs and devices without adequate study of safety and efficacy. Neonates deserve dedicated drug and device development programs, which will require novel approaches and unique collaborations between multiple key stakeholders. Legislative efforts, infrastructure, clinical trial methodology, and international collaborations have all contributed to improvements in neonatal drug and device development, but more work is still needed. Leadership from neonatologists, clinical care providers, and parents is essential to implement needed changes.
Similar content being viewed by others
Introduction
Despite several global initiatives, neonates are often treated with drugs, biologics, and devices that are not approved for use in this population. In addition, there are few new products designed exclusively for neonates. This is due to: (1) gaps in understanding complex pathophysiology, (2) a small market with mostly rare diseases, (3) few appropriate animal models, (4) challenges in the design and execution of trials, and (5) difficulties with the assessment of safety and efficacy due to high rates of co-morbidities and the need for prolonged follow-up. Novel approaches are urgently needed since the smallest neonates may be exposed to multiple drugs (most used off-label) and few innovative devices marketed in the past decade have been approved for pediatric use. In a 2014 review of the 406 medications that were studied in the pediatric population to achieve 6 months of exclusivity, only 28 (7%) had been studied in neonates and most of these were not routinely used in the Neonatal Intensive Care Unit (NICU) [1].
Neonatologists, researchers, regulators, nurses, and parents have all advocated for improved drug and device development. Major areas for advocacy are: (1) promoting legislation that mandates neonatal drug and device studies and streamlines regulatory approvals (e.g., enhanced “breakthrough” designation programs), (2) increasing federal funding for neonatal drug and device development (e.g., Pediatric Trials Network (PTN), Pediatric Device Consortium), (3) new public–private partnerships to develop neonatal drug formulations and novel devices, and (4) using real-world data (RWD) to generate real-world evidence (RWE) to facilitate drug/device approvals. It is also crucial that there is equitable racial/ethnic representation in neonatal clinical trials to enhance the generalizability of results [2, 3]. This manuscript describes current and emerging efforts to improve neonatal drug and device research and to improve the efficiency of regulatory science in this unique population.
Legislative efforts to improve pediatric drug and device development
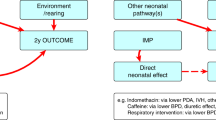
The most urgent need for novel therapies is in extremely preterm neonates who have high rates of morbidity and mortality. Legislative efforts in the US have required the study of drugs, biological and nutritional products, devices, and other therapies through high-quality regulatory and clinical trials, quality improvement initiatives, and observational studies. The Pediatric Research Equity Act (PREA) requires the industry to test the safety and efficacy of new drugs/biologics in children [4]. The Best Pharmaceuticals for Children Act (BPCA) provides financial benefits to companies who voluntarily study new drugs/biologics in children. The FDA Safety and Innovation Act (FDASIA) permanently reauthorized BPCA and PREA and requires neonatal studies to be added to the submitted study plan if relevant and justified. FDASIA also renewed and strengthened the Pediatric Medical Device Safety and Improvement Act (PMDSIA, Fig. 1), with new “breakthrough designation” programs introduced to accelerate product development. The use of alternative study designs, extrapolation, and the potential for the use of RWD represent innovative methods to accelerate new product development in neonates [5].
Neonatal clinical trial infrastructure
Despite exponential increases in new therapeutics/technologies for adults and pediatric patients, neonates still have limited product development. One major success has been the establishment of cooperative neonatal research networks that have offered operational efficiencies including infrastructure, trial support, methodologic advances, and coordination of multicenter research sites. The PTN (https://pediatrictrials.org/) was established by NICHD in response to BPCA and serves as a focal point to pharmaceutical and device trials for children. Over the last decade, PTN has grown to support >100 clinical sites providing scientific, technical, and administrative infrastructure. PTN has advanced methodologies and streamlined processes needed to study medicines efficiently and safely by the use of microblood volumes and advanced pharmacokinetic (PK) modeling methods, minimal sampling techniques, left-over blood specimen analysis, and data mining of electronic health records (EHR) to enhance drug safety information. Recently, PTN partnered with the IDeA States Pediatric Clinical Trial Network to expand research opportunities to underserved and rural communities [6].
The Institute for Advanced Clinical Trials (I-ACT) for Children is an independent non-profit organization dedicated to promoting the development of innovative medical therapies for children (https://www.iactc.org/). As a public–private partnership, I-ACT for Children supports clinical trial networks by bridging pediatric and research institutions, industry sponsors, patient advocacy groups, and others to advance innovation in pediatric research. In Europe, the Pediatric Clinical Research Infrastructure Network developed a toolkit to facilitate the conduct of neonatal clinical trials (https://ecrin.org/projects/pedcrin/pedcrin-tools). The toolkit is comprehensive and addresses all aspects of neonatal clinical trials including trial design, consent, outcome measures, data collection, data analysis, formulations and excipients, and the inclusion of parents in protocol development. They have provided three publicly available webinars which also include one on engagement with families and pediatric patients.
Several international organizations are building global neonatal collaborations with multidisciplinary expertise. The International Neonatal Consortium (INC) works within the pre-competitive space to improve the measurement/assessment of clinical outcomes through data sharing to advance regulatory science. INC addresses the most common medical conditions seen in the NICU by engaging multiple global stakeholders (regulators, industry, investigators, nurses, families, advocacy groups) in discussions to advance drug development processes. The System of Hospitals for Innovation in Pediatrics Medical Devices (SHIP-MD) was formed as the operational arm of the Pediatric Device Consortium to facilitate the development of advanced technologies for children (https://c-path.org/ship-md/). SHIP-MD will: (1) de-risk and accelerate developmental processes, (2) stimulate investment and innovation, and (3) establish predictable regulatory and reimbursement pathways to make more products available for children. Connect 4 Children (c4c) is a large European network designed to facilitate product development for children. The goal of c4c is to establish a sustainable infrastructure to conduct multinational clinical trials, primarily by engaging key stakeholders in the public and private sectors (https://conect4children.org/).
New paradigms in neonatal clinical trial methodology
Improved neonatal outcome through novel therapeutics requires smart, feasible, high-quality clinical trials. These trials need to optimize the use of existing data sources and develop clinically meaningful outcomes (to investigators, families, regulators) while minimizing any potential adverse effects on neonates and their families. A consensus for standardized clinical practices is needed to accurately assess morbidities for neonatal conditions. Innovative study protocols using adaptive, Bayesian, or platform designs and master protocols for multi-drug and multinational studies can reduce the number of neonates in a clinical trial. One example of multi-stakeholder consensus is the published recommendations for the design of therapeutic trials of drugs for neonatal seizures [7]. This publication offered an expert opinion on the essential components for the development of a master protocol, helping researchers conduct more efficient studies while supporting safe, effective, and innovative treatments for neonates. Other attempts to increase efficiency involve the centralization of Institutional Review Boards (IRBs). Neonatal studies involve a vulnerable population, rare diseases, increased morbidity and mortality, and numerous ethical issues. These trials often include time-sensitive enrollment, complicated consent forms, long study durations (including long-term follow-up), and complex endpoints. Multicenter neonatal studies may be delayed by inconsistencies and inefficiencies associated with multiple site local IRB reviews. The NICHD neonatal research network reviewed one clinical trial and found that 50% of IRBs approved the protocol while 50% found major concerns regarding the comparator arm, inclusion criteria, and consent [8]. A central IRB (cIRB) for neonatal studies would provide a streamlined, consistent approach for review and approval of a multicenter neonatal trial. The regulatory requirement for a community person on the IRB panel could include a parent of a neonate. The FDA, NIH, Office Human Research Protections support the use of cIRBs for multicenter studies to improve operational efficiencies of trial conduct [9]. cIRBs also serve to optimize trial start-up times and increase opportunities for patients to participate in research at smaller institutions close to their home. For example, the National Cancer Institute (NCI)-independent central IRB (CIRB, https://www.ncicirb.org/) serves as the IRB of record for all NCI-funded studies ensuring that clinical trials are reviewed efficiently with the highest ethical and quality standards to protect participants. As of January 20, 2020, studies subject to the Revised Common Rule Cooperative Research Provision (45 CFR 46.114(b)) must use a single IRB as required by the terms and conditions of the award. The Central IRB is an entity offered by some academic institutions or commercial entities that may require a fee. The individual site IRBs would need to rely on the Central IRB review, which is already standard with most NIH multicenter studies.
RWD and RWE are increasingly important in drug development, drug safety, and health care decisions, yet how these data will be used to inform neonatal studies is not yet clear [10, 11]. RWD specifically refers to data relating to patient health status and/or the delivery of health care collected from a variety of sources including EHR, claims and billing activities, product and disease registries, and patient care data. RWE is derived from analysis of RWD and can be generated by different study designs including prospective, retrospective, observational, and randomized. The FDA has issued draft guidance for the use of RWD and RWE for drugs, biologics, and devices (https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence). In 2020, the FDA funded a pivotal neonatal project supporting the use of RWD to generate RWE in neonates. This INC/Critical Path Institute project supports the collection and systematic integration of global neonatal data from EHR, national neonatal databases, and clinical trials (https://c-path.org/fda-awards-c-path-grant-to-use-real-world-data-to-generate-real-world-evidence-in-neonates/). These data are being deposited into a RWD and Analytics Platform by data scientists from the Critical Path Institute and could potentially provide a shared global data resource. The first two projects aim to define actionable reference ranges for common laboratory values in neonates and develop natural history disease progression models for bronchopulmonary dysplasia (BPD). The formation of these types of sharable data resources is critical to therapeutic advances and medication safety assessments.
Adverse event (AE) reporting systems provide the foundation for patient safety in clinical trials and knowledge of medication safety. Critically ill neonates have significant rates of AEs, morbidities, and mortality related to intensive care interventions, prematurity, and other disease processes (e.g., birth asphyxia, infection, congenital disorder, etc.). Neonatal trials often report a high incidence of AEs and it can be difficult to distinguish the effects of a clinical illness from an investigational product. Standardization of AE data collection, reporting, and assessment would allow for a better understanding of important medication safety signals within this high background of AEs. International efforts have produced standard terminology for pediatric and neonatal AEs mapped to larger dictionaries such as the Medical Dictionary of Regulatory Activities [12]. Recently, a Neonatal Adverse Event Severity Scale (NAESS) was developed using a Delphi method and may be used by clinicians, neonatal care staff, and investigators to develop capability and consistency in documenting the severity of many AEs routinely encountered in the NICU [13]. Since the NAESS tool has now been independently validated using existing clinical trials data, this approach can be routinely incorporated into neonatal clinical trials [14]. In the future, laboratory-based AEs will be included in the NAESS when age-appropriate reference values become available. These efforts to standardize AE reporting and assessment will lead to more reliable and comparable information to improve safety evaluations of drugs.
Harmonization of consent language, research design for PK/PD-derived dosing recommendations, and clinically meaningful endpoints will help facilitate global efforts to ensure that research outcomes meet regulatory standards and provide meaningful improvements in survival or functional health. Consensus recommendations have described key elements of safety, dosing, and pharmaceutical quality for studies of the medicinal product in neonates [15]. This manuscript has been used by multiple regulatory agencies worldwide to issue guidance with respect to the conduct of neonatal clinical trials. Importantly, the age range for neonates was clarified to include birth to 44 weeks post-menstrual age to account for the impact of prematurity. In addition, a Neonatal Core outcome set of 12 conditions has been proposed as the minimum outcome measures for neonates receiving care in high-resource settings [16]. This minimum dataset includes key items agreed upon by previous patients, parents, nurses, physicians, and researchers. Further details regarding specific measurement tools and timing of measurements are needed to standardize and imbed this core outcome set into trials. Additional efforts have been focused within disease-specific areas such as prevention/treatment of BPD/chronic pulmonary insufficiency of prematurity [17]. This is crucial to better understand natural disease progression, define optimal time points for interventions, and develop clinically meaningful endpoints. These global efforts provide the critical foundation needed to support drug development programs and therapeutic innovations for the neonatal population.
“Team science” and key stakeholder engagement
To accelerate high-quality neonatal trials and support drug/device innovation, multidisciplinary teams should bring together physicians, nurses, parents, researchers, sponsors, funding agencies, regulators, advocacy groups, and biostatisticians with shared data sources. Parents and patient advocacy groups are critical partners in neonatal drug development from the early stages of trial design through trial completion and the dissemination of research results [18, 19]. Families have provided critical insight into inclusion criteria, consent processes, outcome measures, enrollment, participant engagement, retention, and the need for long-term follow-up. Drug development programs that value the voice of children and their families are more likely to succeed. The International Children’s Advisory Network is an international consortium working to elevate the voices of children and families for medicine, research, and innovation. Neonatal nurses and nurse practitioners are also critical partners in neonatal research. Advancing neonatal therapeutics requires input from all key stakeholders invested in improved neonatal care and outcomes.
Clinical pharmacologists provide important input into developmental physiology, pharmacogenomics (e.g., response to specific treatments), and pharmacometrics. Term and preterm neonates represent a vulnerable sub-group of pediatric patients in whom special attention is needed to rationalize dose selection based upon PK/PD and safety studies in neonates. Fortunately, methodologic advances in minimal blood sampling techniques, population PK, and pharmacometric modeling and simulation have made this work more feasible [20, 21]. These techniques facilitate the quantitative evaluation of drug exposures, predict variations in drug exposure with the maturation of organ function, and have helped optimize treatment strategies for growing neonates. Recent advances include the use of physiologically based modeling or machine learning approaches to improve predicted drug exposures in young infants of different age groups [22]. Maximizing a multidisciplinary approach, the PTN recently used these methodologies to predict PK guided dosing of investigational treatments for children hospitalized with COVID-19 [23].
Advances in genetic diagnostics and precision therapeutics
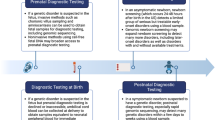
With advancing genetic diagnostics, more patients are diagnosed with rare diseases at early ages and novel therapeutics targeting specific mutations are becoming more available. Several rare diseases are included in newborn screening programs thereby offering very early detection. Families, advocacy groups, and rare disease networks are partnering with industry and researchers to promote drug and device development. Novel precision therapies for cystic fibrosis represent an excellent example of how advocacy and team science can lead to targeted drug development [24]. The Orphan Drug Act of 1983 gave the FDA authority to use federal subsidies and grant protection against generic competition. Advocacy for drug reform has facilitated processes at FDA to expedite the review of treatments for serious conditions and thereby fill unmet medical needs [25]. This includes both financial incentives (delaying generic competition) and specific designations including “fast track, breakthrough therapy, and accelerated approval pathways” that allow surrogate measures of effectiveness while waiting for more definitive research [26]. In 2018, 73% of new drug approvals qualified for expedited review and 50% of new drug approvals were for drugs used to treat rare diseases [27]. Recent studies have indicated that improved diagnostic technologies can be used to identify many neonatal conditions due to rare diseases and genetic disorders [28]. These new methods should be harnessed to introduce therapies earlier (for better long-term outcomes) and to more rapidly advance drug development for rare and genetic diseases in neonates.
It is clear that multiple ongoing efforts to improve neonatal drug and device development are underway. Academic and clinical neonatologists should join with all stakeholders and continue to collaborate and advocate for regulatory studies to support approval for safe and effective therapies to improve the care of neonates globally.
References
Laughon MM, Avant D, Tripathi N, Hornik CP, Cohen-Wolkowiez M, Clark RH, et al. Drug labeling and exposure in neonates. JAMA Pediatr. 2014;168:130–6.
Burris HH, Duncan AF. Rethinking how to persuade more parents from diverse or disadvantaged backgrounds to enroll infants in neonatal clinical trials. JAMA Netw Open. 2021;4:e2032137.
Abdel-Rahman SM, Wimes MP, Curran T. A call to action: issuing a diversity and inclusion challenge to research organizations. Clin Transl Sci. 2021;14:2095–8.
Hwang TJ, Orenstein L, Kesselheim AS, Bourgeois FT. Completion rate and reporting of mandatory pediatric postmarketing studies under the US Pediatric Research Equity Act. JAMA Pediatr. 2019;173:68–74.
Green DJ, Zineh I, Burckart GJ. Pediatric drug development: outlook for science-based innovation. Clin Pharm Ther. 2018;103:376–8.
Annett RD, Chervinskiy S, Chun TH, Cowan K, Foster K, Goodrich N, et al. IDeA states pediatric clinical trials network for underserved and rural communities. Pediatrics. 2020;146:e20200290.
Soul JS, Pressler R, Allen M, Boylan G, Rabe H, Portman R, et al. Recommendations for the design of therapeutic trials for neonatal seizures. Pediatr Res. 2019;85:943–54.
Stark AR, Tyson JE. Inter-center variation in concerns about ethical research design: Neonatal Network experience with Institutional Review Board (IRB) review.• 159. Pediatr Res. 1997;41:29–29.
Flynn KE, Hahn CL, Kramer JM, Check DK, Dombeck CB, Bang S, et al. Using central IRBs for multicenter clinical trials in the United States. PLoS One. 2013;8:e54999.
Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence – what is it and what can it tell us? N Engl J Med. 2016;375:2293–7.
Jarow JP, LaVange L, Woodcock J. Multidimensional evidence generation and FDA regulatory decision making: defining and using “real-world” data. JAMA. 2017;318:703–4.
Gipson DS, Kirkendall ES, Gumbs-Petty B, Quinn T, Steen A, Hicks A, et al. Development of a pediatric adverse events terminology. Pediatrics. 2017;139:e20160985.
Salaets T, Turner MA, Short M, Ward RM, Hokuto I, Ariagno RL, et al. Development of a Neonatal Adverse Event Severity Scale through a Delphi consensus approach. Arch Dis Child. 2019;104:1167–73.
Lewis T, Terrin N, Davis J, Michels K, Salaets T, Wade K, et al. Inter-rater reliability of the Neonatal Adverse Event Severity Scale using real-world Neonatal clinical trial data. J Perinatol. 2021. https://doi.org/10.1038/s41372-021-01164-w. Online ahead of print.
Ward RM, Benjamin D, Barrett JS, Allegaert K, Portman R, Davis JM, et al. Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates. Pediatr Res. 2017;81:692–711.
Webbe JWH, Duffy JMN, Afonso E, Al-Muzaffar I, Brunton G, Greenough A, et al. Core outcomes in neonatology: development of a core outcome set for neonatal research. Arch Dis Child Fetal Neonatal Ed. 2020;105:425–31.
Sheehan S, Baer G, Romine M, Hudson L, Lim R, Papadopoulos E, et al. Advancing therapeutic development for pulmonary morbidities associated with preterm birth. Ther Innov Regul Sci. 2020;54:1312–8.
Vermeulen E, Karsenberg K, van der Lee JH, de Wildt SN. Involve children and parents in clinical studies. Clin Transl Sci. 2020;13:11–13.
Mullin T, Vaidya P, Chalasani M. Recent US Food and Drug Administration efforts to integrate the patient’s perspective in drug development and decision making. Clin Pharm Ther. 2019;105:789–91.
Wilbaux M, Fuchs A, Samardzic J, Rodieux F, Csajka C, Allegaert K, et al. Pharmacometric approaches to personalize use of primarily renally eliminated antibiotics in preterm and term neonates. J Clin Pharm. 2016;56:909–35.
Bi Y, Liu J, Li F, Yu J, Bhattaram A, Bewernitz M, et al. Model-informed drug development in pediatric dose selection. J Clin Pharm. 2021;61:S60–S69.
Tang BH, Guan Z, Allegaert K, Wu YE, Manolis E, Leroux S, et al. Drug clearance in neonates: a combination of population pharmacokinetic modelling and machine learning approaches to improve individual prediction. Clin Pharmacokinet. 2021;60:1435–48.
Maharaj AR, Wu H, Hornik CP, Balevic SJ, Hornik CD, Smith PB, et al. Simulated assessment of pharmacokinetically guided dosing for investigational treatments of pediatric patients with coronavirus disease 2019. JAMA Pediatr. 2020;174:e202422.
Lopes-Pacheco M, Pedemonte N, Veit G. Discovery of CFTR modulators for the treatment of cystic fibrosis. Expert Opin Drug Discov. 2021;16:897–913.
Miller KL, Fermaglich LJ, Maynard J. Using four decades of FDA orphan drug designations to describe trends in rare disease drug development: substantial growth seen in development of drugs for rare oncologic, neurologic, and pediatric-onset diseases. Orphanet J Rare Dis. 2021;16:265.
Pregelj L, Hine DC, Kesselheim AS, Darrow JJ. Assessing the impact of US Food and Drug Administration breakthrough therapy designation timing on trial characteristics and development speed. Clin Pharmacol Ther. 2021;110:1018–24.
New Drug Therapy Approvals. 2018. https://www.fda.gov/files/drugs/published/New-Drug-Therapy-Approvals-2018_3.pdf. Accessed 9/1/2021.
Maron JL, Kingsmore SF, Wigby K, Chowdhury S, Dimmock D, Poindexter B, et al. Novel variant findings and challenges associated with the clinical integration of genomic testing: an interim report of the Genomic Medicine for Ill Neonates and Infants (GEMINI) Study. JAMA Pediatr. 2021;175:e205906.
Author information
Authors and Affiliations
Contributions
TL, KCW, and JMD wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lewis, T., Wade, K.C. & Davis, J.M. Challenges and opportunities for improving access to approved neonatal drugs and devices. J Perinatol 42, 825–828 (2022). https://doi.org/10.1038/s41372-021-01304-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01304-2
This article is cited by
-
Analysis of the first ten years of FDA’s rare pediatric disease priority review voucher program: designations, diseases, and drug development
Orphanet Journal of Rare Diseases (2024)
-
Advocacy in neonatology: current issues and introduction to the series
Journal of Perinatology (2023)