Abstract
Data on outcome in older (≥70 years) patients with acute promyelocytic leukemia after treatment with arsenic trioxide (ATO) compared with standard chemotherapy (CTX) is scarce. We evaluated 433 patients (median age, 73.4 years) treated either with ATO+ all-trans retinoic acid (ATO/ATRA; n = 26), CTX/ATRA + ATO during consolidation (CTX/ATRA/ATO; n = 148), or with CTX/ATRA (n = 259). Median follow-up for overall survival (OS) was 4.8 years. Complete remissions (CR) were achieved in 92% with ATO/ATRA and 82% with CTX/ATRA; induction death rates were 8% and 18%, respectively. For analysis of postremission outcomes we combined the ATO/ATRA and CTX/ATRA/ATO groups (ATO/ATRA ± CTX). Cumulative incidence of relapse (CIR) was significantly lower after ATO/ATRA ± CTX compared with CTX/ATRA (P < 0.001). The same held true when restricting the analysis according to the treatment period after the year 2000. OS of patients in CR1 was not different between ATO/ATRA ± CTX compared with CTX/ATRA (P = 0.20). High (>10 × 109/l) white blood cell (WBC) counts at diagnosis were associated with higher CIR (P < 0.001) compared with lower WBC in the CTX/ATRA group, but not in the ATO/ATRA ± CTX group (P = 0.48). ATO, when added to ATRA or CTX/ATRA is feasible and effective in elderly patients for remission induction and consolidation, particularly in patients with high WBC at diagnosis.
Similar content being viewed by others
Introduction
Upfront arsenic trioxide (ATO) and all-trans retinoic acid (ATRA) combination has been proven to be highly effective in acute promyelocytic leukemia (APL) and has become standard therapy in younger adult, nonhigh-risk patients [1, 2]. Within the pre-ATO era, high white blood cell (WBC) counts were identified as a risk factor for relapse [3,4,5,6]. After initial induction treatment with ATRA and idarubicin (AIDA), subsequent risk-adapted consolidation therapy has shown to partly equalize the risk of relapse between APL-risk groups [5, 7].
To date, reports on outcome of older APL patients (≥70 years) with ATO/ATRA are scarce [8, 9]. Although the approval of ATO in the USA and Europe was based on the randomized APL0406 study in patients 18–70 years of age [1, 2] no upper age limit for the treatment with ATO/ATRA was included in both labels. Thus, clinical data on patients above the age of 70 years treated with different regimens will help clinicians to identify best treatment options for their older APL patients.
In addition, a high mortality before start and during the first day of treatment is still a major issue in APL, particularly in older patients according to the Swedish adult acute leukemia registry [10]. Early death rates after ATRA± anthracycline-based induction therapy were as high as 60% in patients above the age of 80 years and still 18.8% in patients aged 50–59 years [10]. Furthermore, despite dose reduction of chemotherapy (CTX) in older patients [11,12,13] non-relapse mortality during postremission therapy remained high, with 10–18.6% dying mainly due to infections, whereas it was much lower (6.9%) after therapy with ATO [8]. In contrast to CTX/ATRA, only single cases with a secondary malignancy are reported after ATO treatment [8].
The objectives of our study were to evaluate characteristics and outcome of older (≥70 years) APL patients treated with different treatment strategies in a large, international cooperative cohort of patients.
Patients and methods
Patients and treatment
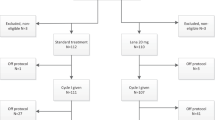
Data on 475 patients aged ≥70 years, reported either to the multicenter, multinational registries German Intergroup Napoleon registry (n = 28), Spanish PETHEMA (Programa Español de Tratamientos en Hematología, n = 211), or French APL Group (n = 228) or to the local data base at the Johns Hopkins University of Maryland, USA (n = 8), were collected in a large international collaboration. All patients were treated between 1990 and 2018. Patients who were treated less intensively with single-agent ATRA or in combination with low-dose cytarabine or comparable CTX (n = 38) or patients with lacking data on response and survival (n = 4) were excluded from analysis. The final study cohort consisted of n = 433 patients. Diagnosis of APL was based on genetic analysis as well as on French–American–British Cooperative Group criteria [14], and, after 2003, on revised International Working Group criteria [15]. Chromosome banding was performed using standard techniques, and karyotypes were described according to the International System for Human Cytogenetic Nomenclature [16]. The diagnosis was confirmed by either reverse-transcriptase polymerase chain reaction (RT-qPCR) or fluorescence in situ hybridization detection by standard methods. FLT3 mutation screening for internal tandem duplications (ITD) and point mutations within the tyrosine kinase domain (TKD) was carried out as previously described [17, 18]. Data collection and analysis were approved by the local Institutional Review Boards.
Treatment
Two-hundred and fifty-nine patients (60%) were treated with ATRA and an anthracycline (daunorubicin or idarubicin) as induction and different CTX in combination with ATRA as consolidation therapy according to treatment protocols active in various institutions and cooperative groups (Supplementary Table 1). These protocols included the PETHEMA LPA96 [19] (n = 23), LPA99 [20] (n = 45), LPA2005 [21] (n = 76), and LPA2012 (ClinicalTrials.gov Identifier: NCT02020161; n = 31), the French APL93 [7] (n = 34) and APL2000 [22] (n = 41) trials, as well as standard AIDA-based CTX [23] (n = 9). Twenty-six (6%) patients were treated with ATO/ATRA [1] (including one dose of idarubicin, n = 1 or daunorubicin for ATRA-syndrome, n = 1; and 5 days of decitabine, n = 1). In addition, 148 (34%) patients were treated with CTX/ATRA + ATO according to the French APL2006 trial [24] (n = 144), or according to Gore et al. [25] (n = 4). Response and treatment failure were assessed according to Cheson et al. [15].
Statistical analyses
Overall survival (OS) was defined as recommended [15]. Induction death was defined as death occurring before complete remissions (CR) evaluation, occurring at the latest within 43 days after treatment initiation in our cohort.
Comparisons of patient characteristics were performed with the Kruskal–Wallis rank sum test for continuous variables and Fisher’s exact test for categorical variables, respectively. The median follow-up time was computed using the reverse Kaplan–Meier estimate [26]. The Kaplan–Meier method was used to estimate the distribution of OS [27]. Confidence interval (CI) estimation for survival curves was based on the cumulative hazard function using Greenwood’s formula for variance estimation. Logrank tests were employed to compare survival curves between groups. For cumulative incidence of relapse (CIR) and death (CID), equality of cumulative incidences between the treatment groups was evaluated using the Gray’s test [28]. A cause-specific Cox proportional hazards regression model was used to identify prognostic variables for relapse [29]. The following variables were included in the Cox model: age at diagnosis, gender, APL treatment (ATO/ATRA ± CTX vs. CTX/ATRA), and WBC count (dichotomized, ≤10 × 109/l vs. >10 × 109/l). All statistical analyses were performed with the statistical software environment R, version 3.3.1, using the R packages, and survival, version 2.39-5 [30].
Results
In total, 433 older APL patients, diagnosed between 1990 and 2015 from four study groups/institutions in the USA and Europe, were included. Median age was 73.4 years (range, 70–89 years).
Risk categorization based on WBC count at diagnosis was available in 429 (99%) of the 433 patients and was low-/intermediate-risk (WBC ≤ 10 × 109/l) in 339 (79%) and high-risk (WBC > 10 × 109/l) in 90 (21%) patients.
Information on cytogenetics was available in 381 (88%) patients. In 26 (7%) of the 381 patients t(15;17) could only be detected by FISH and/or RT-qPCR, whereas cytogenetics showed a normal karyotype. In 238 (67%) of the remaining 355 patients, the balanced t(15;17) translocation was the sole abnormality, whereas in 117 (33%) patients, the translocation was accompanied by additional cytogenetic abnormalities, most frequently trisomy 8 (n = 7; 6%) or t(15;17) within a complex karyotype (≥2 cytogenetic abnormalities in addition to t(15;17); n = 12; 10%).
Regarding FLT3-ITD and TKD mutations, information was available in only 56 (13%) of the 433 patients. Of those, eight (14%) patients had FLT3-ITD and two (4%) had a FLT3-TKD mutation. Patients with FLT3-ITD mutations had significantly higher WBC at diagnosis as compared with FLT3 wild type patients (P < 0.001).
Information on the PML-RARA transcript isoform (breakpoint cluster region (BCR)) was available in 292 patients; 125 (43%) had the short isoform (BCR3). Baseline characteristics according to the frontline strategy are shown in Table 1.
Median age was in trend higher in the ATO/ATRA group as compared with the CTX/ATRA and CTX/ATRA/ATO groups. Median WBC, percentage of peripheral blood and bone marrow blasts as well as risk categorization were significantly lower in the ATO/ATRA ± CTX group as compared with the CTX/ATRA group.
Response to induction therapy
Overall, 356 patients achieved a CR (82%), two patients had refractory disease (0.5%) and 75 patients died during induction therapy (17%). The rate of death during induction therapy was lower in patients receiving ATO/ATRA (n = 2/26; 8%) as compared with those receiving CTX/ATRA (n = 73/407; 18%) as induction therapy (P = 0.28). The rate of deaths during induction therapy decreased over time from 28% (17 of 60 patients) in 1990–1999, to 15% (29 of 188 patients) in 2000–2009 and 16% (29 of 185 patients) in 2010–2018 (P = 0.07). Main causes of deaths were bleeding/hemorrhage (n = 16), infections (n = 17), differentiation syndrome (n = 9), multi-organ failure (n = 3), as well as other causes (not further specified; n = 30).
CRs were achieved after induction therapy in 82% with CTX/ATRA (n = 332/407) and in 92% with ATO/ATRA (n = 24/26; Table 2; P = 0.29). In a logistic regression model on response to induction therapy WBC count above 10 × 109/l (P < 0.001) and age above 75 years (P = 0.04) were unfavorable factors, whereas treatment with ATO/ATRA was a beneficial, but not a significant factor (P = 0.34; Table 3). APL was refractory after CTX/ATRA treatment in two patients, who died after 51 and 477 days from diagnosis, respectively. None of the patients in the ATO/ATRA group was refractory.
Of twelve patients with a complex karyotype, eight were treated with AIDA-based regimen, whereas four patients received ATO/ATRA. Two patients treated with AIDA died after induction therapy and two patients relapsed, whereas no induction death or relapse occurred after ATO/ATRA treatment.
Survival analysis
Median follow-up for survival was 4.8 years (95% CI, 4.3–5.6 years). Overall, the estimated 5-year OS rate was 66% (95% CI, 61–71%). As we were interested in the effect of ATO on CIR and CID, as well as survival of CR patients we combined ATO/ATRA and CTX/ATRA + ATO in one group (ATO/ATRA ± CTX). Treatment with ATO/ATRA ± CTX was associated with significantly reduced CIR after 5 years (4% vs. 18%; P < 0.001; Fig. 1a) with no significant differences in CID (12% and 13%; P = 0.73; Fig. 1b) and OS rates of CR patients (84% (95% CI, 78–91) vs. 77% (95% CI, 71–84%); P = 0.20; Fig. 2) as compared with CTX/ATRA. When restricting the analysis according to the treatment period after the year 2000, ATO/ATRA ± CTX was still associated with significantly lower CIR (P = 0.02) as compared with CTX/ATRA. Of note, higher WBC counts at diagnosis (>10 × 109/l) were associated with an increased CIR in CTX/ATRA (P < 0.001; Fig. 3a), but not in ATO/ATRA ± CTX (P = 0.48; Fig. 3b).
a Cumulative incidence of relapse according to risk category (white blood cell count ≤10 × 109/l vs. >10 × 109/l) in patients treated with all-trans retinoic acid and chemotherapy. b Cumulative incidence of relapse according to risk category (white blood cell count ≤10 × 109/l vs. >10 × 109/l) in patients treated with arsenic trioxide-based regimens.
Overall, five patients relapsed after ATO/ATRA ± CTX (median time from CR to relapse, 18.2 months) as compared with 33 after CTX/ATRA (median time from CR to relapse, 5 months). Of those, risk categorization was high in one patient of the ATO/ATRA ± CTX group as compared with 22 patients of the CTX/ATRA group.
Only two patients experienced besides a hematological a CNS relapse. Both relapses occurred after AIDA-based therapy. Radiotherapy was applied in one patient, whereas the other patient received no treatment. Both patients died shortly after relapse (13 days and 98 days).
Of the CTX/ATRA group eight patients received ATO and additionally five patients ATO/ATRA as salvage therapy. Overall, 12 of the 13 relapsed patients of the CTX/ATRA group achieved a second remission, whereas one patient died during salvage therapy. Multivariate analysis showed that higher WBC count (>10 × 109/l; hazard ratio (HR), 7.90; P < 0.001) had an adverse impact on relapse, whereas therapy with ATO/ATRA ± CTX (HR, 0.23; P = 0.003) was beneficial (Table 4).
Fifty-five patients died in remission (CTX/ATRA, n = 37; ATO/ATRA ± CTX, n = 18), mainly due to infections (n = 12; CTX/ATRA, n = 7 and ATO/ATRA + CTX, n = 5), bleeding/hemorrhage (n = 9; CTX/ATRA, n = 2 and ATO/ATRA + CTX, n = 7), cardiac arrest + aneurysm rupture in the brain (ATO/ATRA + CTX, n = 1), encephalitis/cerebellitis (ATO/ATRA + CTX, n = 2), prostate cancer (ATO/ATRA + CTX, n = 1), and unknown causes (n = 30; CTX/ATRA, n = 28 and ATO/ATRA + CTX, n = 2).
Among patients achieving CR after intensive therapy, the median follow-up time was 3.4 years for ATO/ATRA ± CTX and 7.2 years for CTX/ATRA. Data on the development of secondary neoplasms were available in 163 patients. Of those, 11 (7%) patients developed a secondary neoplasm (gastro-intestinal cancer, n = 4; lung cancer, n = 3; neuroendocrine carcinoma, n = 1; and secondary acute myeloid leukemia, n = 3) after a median of 61.5 months (range, 5.5–85 months) from the time point of CR. All of them had been treated with CTX/ATRA. In a competing risk analysis among patients in CR after intensive therapy, the rate of secondary malignancies was significantly lower after ATO/ATRA ± CTX as compared with CTX/ATRA (P = 0.02).
Discussion
Our retrospective analysis of a large cohort of 433 older APL patients, spanning a time period of almost 28 years, shows a higher response rate after treatment with ATO/ATRA as compared with CTX/ATRA, primarily due to a lower rate of induction deaths, which is in line with published data in younger APL patients [1, 9]. The rate of induction deaths decreased from 28% between 1990 and 1999 to 15% and 16% of the following decades, which may be attributable to improved supportive care and awareness of APL as a medical emergency. This finding suggests that the clear guidance in international recommendations about early supportive care has had an effect in recent years [31]. Thus, an effective strategy to overcome induction mortality is due to standardized guidelines along with consultative support and sharing of expertise [32]. Main causes of induction death after CTX/ATRA included bleeding/hemorrhage and infections, which is in line with previously published data [10, 33, 34]. However, 30 patients experienced induction death due to other causes, which might simply be related to the fact, that older patients might have more frequent and severe comorbidities and thus lower capacity to tolerate CTX [10]. The main limitation of CTX/ATRA treatment is the high rate of induction death, whereas treatment with ATO/ATRA seems to be safer and being associated with a low-induction death rate. Thus, treating patients with ATO/ATRA during induction seems to be rational rather than in consolidation, when the greatest risk has passed [35]. However, in our cohort the number of patients treated with ATO/ATRA during induction was very low and induction mortality was high after CTX/ATRA, particularly in the earlier treatment period before 1999. Therefore, a comparison of OS between the CTX/ATRA and ATO/ATRA groups is hampered. Thus, we focused in time-to-event analyses on patients in first CR.
As a potential selection bias, none of the patients treated with ATO/ATRA in induction therapy had high risk (WBC count >10 × 109/l) at diagnosis as compared with 28% in the CTX/ATRA group, which is a known risk factor for bleeding diathesis [3]. In addition, as a further potentially selection bias, percentage of peripheral blood and bone marrow blasts were also unequally distributed between the CTX/ATRA and ATO-based groups.
Regarding postremission outcome, results of the randomized North American Leukemia Intergroup Study C9710 on 481 APL patients (age range, 15–79 years including 77 patients above the age of 60 years) evaluating ATO in first-line therapy during consolidation demonstrated that ATO further reduced the risk of relapse and improved survival as compared with consolidation with daunorubicin/cytarabine [35]. In contrast, a subgroup analysis of the randomized phase-III AML17 trial of the UK National Cancer Research Institute Acute Myeloid Leukaemia Working Group on 49 older (age range, ≥60–77 years) patients showed no significant difference of the 4-year OS rate after treatment with ATO/ATRA (74%; n = 25) as compared with the AIDA-based regimen (80%; n = 24) [9]. However, this was in contrast to the total study cohort with significantly better event-free and relapse-free survival after ATO/ATRA as compared with the AIDA-based regimen. In our cohort postremission outcomes were comparable after CTX/ATRA and ATO-based treatment in patients with low-/intermediate-risk, which is in contrast to previously published data in younger adults [1, 2]. Contrary to the data of the AML17 trial [9], the ATO-based treatment resulted in significant lower CIR in patients with high-risk.
There was no difference between CTX/ATRA and ATO-based regimens with regard to deaths in remission with relatively high rates of 12% and 13%, which is probably due to the advanced age of the cohort. Besides bleeding infections were the most frequent cause of death, particularly after CTX/ATRA treatment, arguing for a CTX-free approach. Other causes were most probably related to the natural death rate.
In addition, there was a significant difference in the occurrence of secondary malignancies, since none of the patients developed a secondary therapy-related myeloid neoplasm or solid tumor after ATO-based regimens as compared with 11 (7%) patients after CTX/ATRA, including four patients, who developed a secondary malignancy within 2.5 years after achievement of CR. Although the median follow-up time was shorter after ATO-based regimens as compared with CTX/ATRA, the reported latency period between APL diagnosis and the development of a secondary malignancy of 6.6 months strongly argues for a true lower incidence after ATO/ATRA ± CTX [36]. The low-relapse rate after ATO-based therapy in our cohort is in line with others [8, 35]. Moreover, 12 relapsed patients of the CTX/ATRA group were successfully salvaged with ATO-based therapy, confirming the tolerability of this treatment option in elderly patients. In contrast to the publication of Powell et al. [35] presenting a subgroup analysis in older patients, treatment with ATO/ATRA was not superior to the standard CTX/ATRA-approach in the low-/intermediate-risk in our cohort. However, a clear and significant benefit was seen in the high-risk group. Thus, our data suggest that ATO-based regimens are effective in older patients independent of risk classification.
Regarding biological characteristics, additional cytogenetic abnormalities were present in 25% of the patients, most frequently trisomy 8, which is in line with published data [37, 38]. In addition, t(15;17) could also be found within a complex karyotype, suggesting that older age might lead to higher chromosomal instability and thus higher rate of abnormalities, as has been described [39]. Although data were limited to a low number of patients, FLT3-ITD mutations seem to be less frequent in older (14%) as compared with all APL patients (31%) [35]. As expected, FLT3-ITD mutated patients had significantly higher WBC count at diagnosis as compared with FLT3 wild type patients [37, 40,40,41,42,43]. To date, there are still conflicting data regarding the impact of additional chromosomal or genetic abnormalities on outcome in APL patients [37, 40,41,42,43,44,45,46,47,48,49]. In our large cohort, the presence of additional cytogenetic abnormalities had no impact on OS (P = 0.99), whereas FLT3-ITD was associated with an adverse impact on OS (P < 0.001). The latter issue, however, should be interpreted with precaution due to the low availability of FLT3 mutational status in our cohort.
Regarding the distribution of risk category according to WBC count at diagnosis, published data are again contradictory [49, 50]. Sanz et al. [49] reported that older patients seem to be more likely to present with non-high-risk APL as compared with their younger counterparts (37% vs. 18%), which in part may account for the low relapse rate observed in their publication. In contrast, Lengfelder et al. reported on 31% (n = 28/91) of older patients with high-risk APL [50]. In our cohort, the proportion of high-risk patients in the CTX/ATRA group was comparable with the latter publication [50], whereas it was lower in the ATO/ATRA/ ± CTX group due to study inclusion criteria [24] as well as reservation to use ATO-based therapy in high-risk patients. As expected, higher WBC count above 10 × 109/l was an unfavorable factor for response to induction therapy as well as CIR and CID. Of note, no difference in outcome was observed in high-risk patients after treatment with ATO/ATRA ± CTX, whereas this was not the case in patients after treatment with CTX/ATRA, suggesting that the ATO-based regimen is also highly active within the high-risk group.
In conclusion, ATO, when added to ATRA or CTX/ATRA, is a feasible and efficacious treatment and leads to good outcomes in the primary management of older APL patients. Thus, it seems to be prudent to expand this approach to older APL patients.
Change history
16 November 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41375-021-01358-3
References
Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–21.
Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2016;35:605–12.
Testa U, Lo-Coco F. Prognostic factors in acute promyelocytic leukemia: strategies to define high-risk patients. Ann Hematol. 2016;95:673–80.
Sanz MA, Lo Coco F, Martín G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–53.
Sanz MA, Martin G, Gonzalez M, et al. Risk-adapted treatment of acute promyelocytic leukemia with alltrans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103:1237–43.
Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94:1192–200.
Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116:3171–9.
Zhang Y, Zhang Z, Li J, et al. Long-term efficacy and safety of arsenic trioxide for first-line treatment of elderly patients with newly diagnosed acute promyelocytic leukemia. Cancer. 2013;119:115–25.
Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:1295–305.
Lehmann S, Ravn A, Carlsson L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25:1128–34.
Ades L, Chevret S, De Botton S, et al. Outcome of acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy in elderly patients: the European group experience. Leukemia. 2005;19:230–3.
Mandelli F, Latagliata R, Fazi P, Rodeghiero F, Leoni F, et al. Treatment of elderly patients (> or =60 years) with newly diagnosed acute promyelocytic leukemia. Results of the Italian multicenter group GIMEMA with ATRA and idarubicin (AIDA) protocols. Leukemia. 2003;17:1085–90.
Disperati P, Minden MD, Gupta V, et al. Acute promyelocytic leukemia in patients aged 70 years and over—a single center experience of unselected patients. Leuk Lymphoma. 2007;48:1654–8.
Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French–American–British Cooperative Group. Ann Intern Med. 1985;103:620–5.
Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–9.
Mitelman F. ISCN: an international system for human cytogenetic nomenclature. Basel: S. Karger; 1995.
Yokota S, Kiyoi H, Nakao M, et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia. 1997;11:1605–9.
Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35.
Sanz MA, Martín G, Rayón C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalphapositive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94:3015–21.
Sanz MA, Montesinos P, Vellenga E, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA Group. Blood. 2008;112:3130–4.
Sanz MA, Montesinos P, Rayon C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high risk patients: further improvements in treatment outcome. Blood. 2010;115:5137–46.
Adès L, Chevret S, Raffoux E, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24:5703–10.
Mandelli F, Diverio D, Avvisati G, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell’Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 1997;90:1014–21.
Rahmé R, Ades L, Thomas X, et al. Reducing mortality in newly diagnosed standard-risk acute promyelocytic leukemia in elderly patients treated with arsenic trioxide requires major reduction of chemotherapy: a report by the French Belgian Swiss APL group (APL 2006 trial). Haematologica. 2018;103:e519–21.
Gore SD, Gojo I, Sekeres MA, et al. Single cycle of arsenic trioxide-based consolidation chemotherapy spares anthracycline exposure in the primary management of acute promyelocytic leukemia. J Clin Oncol. 2010;28:1047–53.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6.
Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;168:1141–54.
Cox DR. Regression models and life tables (with discussion). J R Stat Soc. 1972;34:187–220.
R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014.
Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–91.
Jillella AP, Kota VK. The global problem of early deaths in acute promyelocytic leukemia: a strategy to decrease induction mortality in the most curable leukemia. Blood Rev. 2018;32:89–95.
Martínez-Cuadrón D, Montesinos P, Vellenga E, et al. Long-term outcome of older patients with newly diagnosed de novo acute promyelocytic leukemia treated with ATRA plus anthracycline-based therapy. Leukemia. 2018;32:21–9.
de la Serna J, Montesinos P, Vellenga E, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;11:3395–4302.
Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116:3751–7.
Pagano L, Pulsoni A. Second malignancy after treatment of acute promyelocytic leukemia: experience of GIMEMA trials. Blood. 2002;100:1514–5.
Schlenk RF, Germing U, Hartmann F, et al. High-dose cytarabine and mitoxantrone in consolidation therapy for acute promyelocytic leukemia. Leukemia. 2005;19:978–83.
Lou Y, Suo S, Tong H, et al. Characteristics and prognosis analysis of additional chromosome abnormalities in newly diagnosed acute promyelocytic leukemia treated with arsenic trioxide as the front-line therapy. Leuk Res. 2013;37:1451–6.
Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–20.
Kiyoi H, Naoe T, Yokota S, et al. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho). Leukemia. 1997;11:1447–52.
Noguera NI, Breccia M, Divona M, et al. Alterations of the FLT3 gene in acute promyelocytic leukemia: association with diagnostic characteristics and analysis of clinical outcome in patients treated with the Italian AIDA protocol. Leukemia. 2002;16:2185–9.
Shih LY, Kuo MC, Liang DC, et al. Internal tandem duplication and Asp835 mutations of the FMS-like tyrosine kinase 3 (FLT3) gene in acute promyelocytic leukemia. Cancer. 2003;98:1206–16.
Lucena-Araujo AR, Kim HT, Jacomo RH, et al. Internal tandem duplication of the FLT3 gene confers poor overall survival in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline-based chemotherapy: an International Consortium on Acute Promyelocytic Leukemia study. Ann Hematol. 2014;93:2001–10.
De Botton S, Chevret S, Sanz M, et al. Additional chromosomal abnormalities in patients with acute promyelocytic leukaemia (APL) do not confer poor prognosis: results of APL 93 trial. Br J Haematol. 2000;111:801–6.
Cervera J, Montesinos P, Hernández-Rivas JM, et al. Additional chromosome abnormalities in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Haematologica. 2010;95:424–31.
Pantic M, Novak A, Marisavljevic D, et al. Additional chromosome aberrations in acute promyelocytic leukemia: characteristics and prognostic influence. Med Oncol. 2000;17:307–13.
Poiré X, Moser BK, Gallagher RE, et al. Arsenic trioxide in front-line therapy of acute promyelocytic leukemia (C9710): prognostic significance of FLT3 mutations and complex karyotype. Leuk Lymphoma. 2014;55:1523–32.
Cicconi L, Divona M, Ciardi C, et al. PML-RARα kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016;30:1987–92.
Sanz MA, Vellenga E, Rayón C, et al. All-trans retinoic acid and anthracycline monochemotherapy for the treatment of elderly patients with acute promyelocytic leukemia. Blood. 2004;104:3490–3.
Lengfelder E, Hanfstein B, Haferlach C, et al. Outcome of elderly patients with acute promyelocytic leukemia: results of the German Acute Myeloid Leukemia Cooperative Group. Ann Hematol. 2013;92:41–52.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SK, PM, and RFS were responsible for the concept of this paper, contributed to the literature search data collection, analyzed and interpreted data, and wrote the paper. UP and LA contributed patients, interpreted data and critically revised the paper. RR, DMC, GG, XT, MS, AGB, AG, AP, CG, ER, MT, NV, JS, OS, EL, MJL, PF, and MAS contributed patients and critically revised the paper. All authors reviewed and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
SK was supported by the Olympia-Morata fellowship program from the Medical Faculty of the Heidelberg University. MJL is supported by a grant from the NCI (NCI Leukemia SPORE P50 CA100632). UP has received research support from TEVA. All other authors declare no competing conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kayser, S., Rahmé, R., Martínez-Cuadrón, D. et al. Outcome of older (≥70 years) APL patients frontline treated with or without arsenic trioxide—an International Collaborative Study. Leukemia 34, 2333–2341 (2020). https://doi.org/10.1038/s41375-020-0758-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0758-4
This article is cited by
-
Incidence, risk factors, and outcomes of second neoplasms in patients with acute promyelocytic leukemia: the PETHEMA-PALG experience
Annals of Hematology (2024)
-
Acute promyelocytic leukaemia: population-based study of epidemiology and outcome with ATRA and oral-ATO from 1991 to 2021
BMC Cancer (2023)
-
All-trans Retinoic Acid, Arsenic Trioxide, and Anthracycline-based Chemotherapy Improves Outcome in Newly Diagnosed Acute Promyelocytic Leukemia Regardless of FLT3-ITD Mutation Status
Current Medical Science (2021)
-
Second cancers in adults with acute promyelocytic leukemia treated with or without arsenic trioxide: a SEER-medicare analysis
Leukemia (2020)