Abstract
Early life stress (ELS), such as abuse and neglect during childhood, can lead to psychiatric disorders in later life. Previous studies have suggested that ELS can cause profound changes in gene expression through epigenetic mechanisms, which can lead to psychiatric disorders in adulthood; however, studies on epigenetic modifications associated with ELS and psychiatric disorders in adolescents are limited. Moreover, how these epigenetic modifications can lead to psychiatric disorders in adolescents is not fully understood. Commonly, DNA methylation, histone modification, and the regulation of noncoding RNAs have been attributed to the reprogramming of epigenetic profiling associated with ELS. Although only a few studies have attempted to examine epigenetic modifications in adolescents with ELS, existing evidence suggests that there are commonalities and differences in epigenetic profiling between adolescents and adults. In addition, epigenetic modifications are sex-dependent and are influenced by the type of ELS. In this review, we have critically evaluated the current evidence on epigenetic modifications in adolescents with ELS, particularly DNA methylation and the expression of microRNAs in both preclinical models and humans. We have also clarified the impact of ELS on psychiatric disorders in adolescents to predict the development of neuropsychiatric disorders and to prevent and recover these disorders through personalized medicine.
Similar content being viewed by others
Introduction
Early life is a highly sensitive and critical period for brain development, cognitive maturation, and the formation of synaptic structures and synaptic interconnections in the central nervous system (CNS) [1]. Adverse experiences in this period may profoundly impact these processes and can lead to psychiatric disorders in adult life. In the USA, more than 180,000 child abuse cases were reported in 2017 [2]. Early life stress (ELS), such as physical abuse, sexual abuse, emotional abuse, physical neglect, and emotional neglect, are known as significant risk factors for many adverse health problems during adulthood [3,4,5] and are associated with physical illnesses such as diabetes, cardiovascular disease, and malignant tumor [6,7,8]. Exposure to ELS is a significant risk factor for mental illnesses such as major depressive disorder (MDD), schizophrenia, bipolar disorder, borderline personality disorder, posttraumatic stress disorder (PTSD), and substance use [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. The number of early life adverse experiences is also correlated with the increased risk of depression during adulthood. For example, there is a 4-times higher risk of depression in a person who had multiple episodes of early-life adversity than in someone who had not experienced any early-life adversity [25].
In childhood and adolescence, ELS is not only associated with the heightened risk of adverse health problems [26,27,28,29,30,31,32], including physical illnesses such as headache [33] and asthma [34] but also mental illnesses such as depression, non-suicidal self-injury (NSSI) [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. A meta-analysis that examined the association between ELS and depression in adolescents found that the association between childhood abuse/neglect and depression was much stronger in adolescents than in adults [50]. More recently, another meta-analysis performed by LeMoult et al. [51] estimated the associations between ELS and the risk for the onset of MDD before the age of 18 years. These authors also examined the associations between a specific type of ELS and risk for youth-onset MDD. They found that individuals who experienced ELS were more likely to develop MDD before the age of 18 years than individuals without a history of ELS. Whereas some types of ELS were not associated with MDD, other types (emotional abuse) were more strongly associated with MDD. It is also suggested that the impact of ELS on adolescents is sex-dependent. For example, a study reported that in young adulthood, the mental health of females with ELS was worse than males with ELS, and males with ELS were associated with more substance use than females with ELS [52].
Suicidal behavior, such as suicide attempts and suicide ideation, is one of the most important psychiatric problems. Affective temperamental dysregulation might be a possible contributor to adverse clinical outcomes in depressed patients [53]. In this context, it is important to note that ELS is significantly associated with suicide attempts [54, 55], suicide ideation [56, 57], and a high risk of premature mortality, including completed suicide [58] later in life. Among various early life stressors, sexual abuse has been strongly associated with suicidal behavior [59, 60] during adulthood. In addition, childhood physical abuse and witnessing domestic violence are significantly linked with a higher risk for both suicidal ideation and attempts [61] in adults. Other studies have also shown an association between ELS and suicide attempts with various psychiatric disorders [62,63,64,65]. Interestingly, patients with mental illnesses who had a history of ELS are a biologically and clinically different subtype with greater symptom severity, poorer treatment outcome, and greater risk for suicide compared to patients who did not have a history of ELS [66]. A recent meta-analysis reported that emotional abuse, physical abuse, and sexual abuse were significantly associated with an elevated risk for suicide attempts in adults [67]. These studies suggest that a specific type of ELS could be specifically associated with suicidal behavior in the adult population.
As with the adult population, ELS is also linked with suicidal behavior in adolescents [68]. Interestingly, females with ELS were more significantly associated with suicide attempts than males with ELS in their early teens [69]. Stratifying the sex differences, one study reported that physical, emotional, and sexual abuse, household dysfunction, psychological symptoms, and lower social support were significantly correlated with suicide attempts in both boys and girls [69]. However, being younger age, and having physical and emotional neglect experiences increased the risk of suicide attempts in girls only. When exposed to physical abuse, being younger age and having lower social support, girls were more likely to have suicide attempts than boys. Conversely, suicide attempts were more among boys than girls when exposed to psychological symptoms. This suggests that the effect of ELS could be sex-specific. Whether this association is specific to the younger population or common to adults, needs to be further studied.
Given the prevalence and substantial association of ELS with depression and suicidal behavior in adolescence, there is an urgent need to identify neurobiological factors that can contribute to these disorders in youth. In this review, we have critically evaluated the current knowledge of genetic and epigenetic factors that can contribute to the development of depression in the adolescent/youth population. Emphasis has been given to epigenetic factors, given that they are significantly affected by the environment. In addition, we have also discussed the possibility of epigenetic modifications being used as effective targets for diagnostic biomarkers or therapy.
Genetic contributors of ELS-induced depression in adolescents
One of the most common studies in ELS is the study of single-nucleotide polymorphisms (SNPs). Many previous studies have reported an association between brain-derived neurotrophic factor (BDNF) and psychiatric disorders such as depression and suicide in the adult population [70,71,72]. However, no significant differences were found in BDNF levels in adolescents with sexual abuse with or without PTSD [73]. Interestingly, in patients with PTSD, decreased cortisol levels were found with increasing time after trauma, and no significant correlation was found with the cortisol levels in patients without PTSD, suggesting that cortisol may play a role in individuals who sustained sexual assault. In the BDNF gene, the SNP, which is the substitution from valine (Val) to methionine (Met) in the functional coding region at codon 66 (BDNF Val66Met), has received the most attention in mental disorders, including depression [74]. Several studies have shown an association between BDNF Val66Met polymorphism and stress responses of ELS [75,76,77,78,79,80,81,82,83,84] and PTSD [85] in adolescents. A study by Chen et al. [84] in 780 pairs of ethnic Han Chinese adolescent twins showed that the frequency of stressful life events was significantly correlated with depressive symptoms, but there was no significant main effect on the BDNF Val66Met genotype. On the other hand, the interaction between the BDNF Val66Met genotype and stressful life event frequency was significant. Individuals with one or two Val alleles demonstrated a greater susceptibility to both the detrimental effects of higher stress and the beneficial effects of lower stress compared to the Met/Met genotype. Another study followed 889 mothers and their children from 3 months to 12 years and examined an association between BDNF Val66Met and behavior problems in adolescents [86]. Information on maternal depressive symptoms was gathered postpartum and at a 12-year follow-up. The results showed a significant association between maternal symptoms of depression and anxiety and the tendency to internalize problems in 12-year-old children. Surprisingly, maternal depressive and anxious symptoms, such as pre-maternal stress, were not associated with BDNF Val66Met and behavior problems in adolescents.
The SNPs of the promoter region of the serotonin transporter gene (5HTTLPR) have not only been studied in adolescent depression with ELS [76, 81, 86] but also with suicidal behavior [87]. In adolescent depression, a significant main effect and a gene-environment interaction effect of the short (SS) allele were found only among females [88], suggesting sex differences in interaction effects between the 5HTTLPR polymorphism and maltreatment in the prediction of adolescent depression. The role of stress, social support, and the short allele of the 5-HTTLPR and the met allele of the BDNF have also been studied in children following a natural disaster [85]. BDNF analyses showed several gene × environment interactions. Greater stress was related to more symptoms of PTSD and depression, and this effect was stronger in children with the met allele. No changes were found for 5-HTTLPR. Some studies have also reported other genes, such as the mineralocorticoid receptor gene (NR3C2) [89], the promotor of serotonin transporter gene (SLC6A4) [82], and FK506-binding protein 5 (FKBP5) [90, 91] with ELS and depression.
As discussed above, a majority of the studies have taken a candidate gene approach. Most of these have focussed on BDNF gene and, to a certain extent, stress-related genes. To the best of our knowledge, there is no meta-analysis that has analyzed the associations between ELS, epigenetics, and adolescent depression or suicide. Candidate gene studies generally focus on known genes that are thought to be involved in the pathogenesis of the disease and compare the frequency of the gene polymorphisms between patients with the disease and controls. These are generally hypothesis-driven, and the advantage is that it allows to compare the findings accumulated by various studies to consider the direction of the research and to accumulate more data steadily. However, biases can easily arise with candidate gene studies. On the other hand, genome-wide association studies (GWAS) cover almost the whole genome and statistically examine the association between SNP frequencies and the disease. These studies are data-driven without a hypothesis in advance, analyze the results, and then proceed with the study to identify SNPs or other variants in the DNA that are associated with the disease. So far, there is no GWAS examining the association between ELS and adolescent depression. Future studies should be directed in this context. In addition, recent molecular genetic studies indicate that the genetic basis for complex traits such as MDD is polygenic, which results from a combination of many genetic variants [92]. Usig a polygenic approach, a recent study examined the interaction between genetic risk for MDD and a multi-informant longitudinal index of critical parenting in relation to depressive symptom development from early to late adolescence [93]. The Latent Growth Model suggested that polygenic risk score for MDD was associated with higher depressive symptoms across adolescence, particularly for those experiencing elevated levels of critical parenting. The authors highlighted how polygenic risk, in combination with a general environmental factor, can influence depressive symptom development from early to late adolescence. Similar approaches need to be used with respect to ELS and depression in both adults and adolescents and examine how they are commonly associated or differ from each other in the development of MDD in these two populations.
Epigenetics and their association with ELS and depression in adolescents
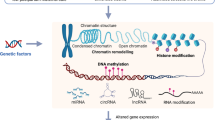
Epigenetics in the context of ELS and psychiatric illnesses during adolescence is an emerging field of research. Epigenetics refers to long-standing gene expression changes regulated via transcriptional, post-transcriptional, translational, and/or post-translational mechanisms such as DNA methylation, DNA hydroxymethylation, and histone modifications, which do not entail any change in DNA sequence. These epigenetic changes have been widely reported in various psychiatric conditions, including suicidal behavior [71, 94,95,96], as well as a biomarker in treatment response [97]. Several recent reviews have highlighted the importance of epigenetics in ELS-induced behavioral changes in humans and animals [6, 98,99,100,101,102,103,104]. A schematic representation of the impact of ELS on genome organization and gene regulation is depicted in Fig. 1. In this review, we aimed to evaluate the current evidence on epigenetic modifications in adolescents with ELS and to explore the possibility of them being used as effective targets for diagnostic biomarkers or therapy.
Early life stress such as physical abuse, sexual abuse, emotional abuse, and neglect leads to epigenetic modifications such as DNA methylation and abnormal microRNA expression, which causes genome reorganization and alterations in gene regulation in a sex-dependent manner. These changes may affect neural networks leading to aberrant cell proliferation, synaptic plasticity, neurotransmission, and neuroinflammation. This may lead to physical and mental illnesses in adolescents and adulthood. Clinical manifestations in adolescents can cause further epigenetic modifications and affect neuropsychiatric disorders in adulthood. G × E gene and environmental, MDD major depressive disorder, miRNA microRNA, PTSD posttraumatic stress disorder, BPD borderline personality disorder.
DNA methylation
DNA methylation is one of the most studied epigenetic mechanisms regarding psychiatric illnesses. DNA methylation, defined as the addition of the methyl group on the fifth carbon of cytosines (5-methyl-cytosine (5mC)), commonly occurs in life, including the development process. DNA methylation is reported at CpG sites at gene promoters and is associated with transcription silencing in mammalian genomes [101]. The functions of 5mC methylation in different regions of the genome, except for the promoter regions, are not fully understood [101]. Early studies focused on CpG islands (CGI) representing DNA regions of a high CpG density, which were shown to be low or unmethylated. Recent work, however, has shown that DNA methylation can also directly silence genes with non-CGI promoters. In DNA methylation at promoter regions, three processes are involved: de novo DNA methylation, maintenance, and demethylation [105]. DNA methyltransferase enzymes transfer the methyl group from S-adenosyl-L-methionine to cytosine, whereas Ten-eleven-translocation proteins methylcytosine dioxygenases and thymine DNA glycosylase execute active demethylation. In the demethylated gene body, CG-rich promoter, which is highly methylated, is silenced [99, 104, 105].
A few studies suggest that DNA methylation plays a crucial role in adolescent depression. For example, in 25 adolescents and 20 healthy controls, Chiarella et al. [106] examined resting-state assessments and brain morphometry along with salivary SCL6A4 and FKBP methylation. They found that SCL6A4 methylation was linked to amygdala-frontal operculum resting-state functional connectivity, regardless of diagnosis, and was differentially associated with inferior orbitofrontal gyrus gray matter volume in adolescents with depression and control subjects. On the other hand, FKBP5 methylation was associated with inferior orbitofrontal gyrus gray matter volume in depressed and healthy adolescents and orbitofrontal cortex rostral prefrontal cortex connectivity only in healthy adolescents. These data show that FKBP5 and SLC6A4 methylation levels are associated with brain connectivity and structure in regions relevant to adolescent depression. Raffetti et al. [107] reported glucocorticoid receptor (NR3C1) DNA methylation was significantly associated with a higher risk for substance use in adolescents, and NR3C1 exon 1F locus hypermethylation can predict substance use in adolescents. Roberson-Nay et al. [108] investigated DNA methylation from 150 Caucasian monozygotic adolescent twins with or without major depression. About 760 differentially and variably methylated probes/regions mapped to 428 genes with early-onset depression were detected. Genes previously implicated in mood and psychiatric disorders, as well as chronic stress (e.g., NRG3) were also identified. Gene enrichment analyses implicated genes related to neuron structures and neurodevelopmental processes, as well as cell adhesion.
Although limited in number, DNA methylation studies are emerging and seem to be critical in adolescent depression. How they are different from adult depression, remains to be seen. Future follow-up studies with repeated measures from childhood to adulthood in individuals with a depressive disorder are needed to understand how the relationship between DNA methylation and brain processes changes throughout the lifespan.
Studies of DNA methylation in adolescents with ELS
Animal studies
Animals have been extensively used to investigate the effects of ELS on epigenetic modifications. Maternal separation (MS) is one of the most popular ELS models [109,110,111,112,113]. In this model, neonatal pups are separated from their mothers for a certain period of time every day for the first 2 to 3 weeks [6]. This results in inconsistent maternal care provided to the pups, which ultimately leads to long-term anxiety-like and depressive-like behaviors. Jaric et al. [114] used C57BL/6 mice to establish a two-hit developmental stress model, including MS in early life followed by social isolation in adolescence. They found that single exposure to early-life stress had the most significant impact and was female-specific in generating anxiety- and depression-related phenotypes. They also reported that transcriptional and methylation alterations in psychiatric risk genes, Nr3c1, and calcium voltage-gated channel subunit alpha1C (Cacna1c), most likely contributed to the stress-induced behavioral effects in these animals. Leussis et al. [115] used the triadic model of learned helplessness to understand controllability, helplessness, and motivational factors following maternal separation in male and female adolescent rats. They found sex-dependent changes, with males demonstrating loss of controllability in an escapable shock condition, whereas females demonstrating motivational impairment in a no-shock condition. Although no epigenetic studies were done in these animals, the authors noted reductions in parvalbumin, a GABAergic marker, in the prefrontal cortex of separated rats relative to age-matched controls and paralleled depressive-like behavior. This model seems promising and can be used to further study epigenetic consequences during adolescence.
Chronic mild stress has also been used as an ELS model [116, 117]. Deng et al. [118] investigated Sprague Dawley rats who received predictable chronic mild stress in adolescence. The study revealed that DNA methylation in exons IV and VI of BDNF were significantly decreased compared to the control. Yang et al. [119] investigated FKBP5 DNA methylation in the hippocampus from C57BL/6J mice, that were administered corticosterone from 5 weeks old for four weeks, as a stress model and revealed intron5 of FKBP5 DNA methylation was significantly decreased compared to controls. In male mice, maternally separated for four hours a day from P10 to P17 combined with limited nesting material, Kronman et al. [120] recently reported downregulation of H3K79me2 in dopamine D2 median spiny neurons of the nucleus accumbens along with altered expression of DOT1 like histone lysine methyltransferase (Dot1L) and lysine demethylase 2b (Kdm2B), enzymes that control this modification.
Recently, Fitzgerald et al. [121] used a modified maternal separation (MMS) model, which involved repeated stimulation of pups for 1.5 h/day, while separated from their mother from postnatal day (P) 4-6. 3′mRNA and DNA methylation immunoprecipitation sequencing were performed on hypothalamic tissue at P6. The authors found that although MMS was associated with subtle changes in gene expression, there were widespread alterations in DNA methylation along with hyperactivity in the elevated plus and open field mazes. They concluded that ELS had marked effects on DNA methylation in the hypothalamus in early life, resulting in stress-specific hyperactivity in young adulthood. In another study, Seo et al. [122] examined the effects of ELS on hippocampal S100 calcium-binding protein A10 (p11) expression, histone acetylation, and DNA methylation at the p11 promoter at different stages of adulthood. Pups were subjected to MS for 3 h daily from postnatal day 1 to 21. At young and middle adulthood, behavioral tests were measured. Mice in both age groups showed reduced hippocampal p11 levels, a decrease in histone acetylation (AcH3), and permissive histone methylation (H3K4me3) at the p11 promoter, as well as an increase in repressive histone methylation (H3K27me3). In addition, AcH3 and H3Kme3 levels of the p11 gene in response to MS were reduced with age. DNA methylation analysis of the p11 promoter revealed increased CpG methylation in middle-aged MS mice only. The results show the age-dependent negative effects of ELS on the epigenetic modifications of p11 transcription.
Altogether, the animal studies demonstrate that regardless of the animal models used, ELS can have widespread epigenetic effects during the adolescent phase; some of them are correlated with behavioral responses. Since animal studies have used rats of different strains as well as different models of ELS, more replication studies are needed. Furthermore, epigenetic modifications vary with brain regions, and some of the changes are cell type-specific; further studies will be required to examine these epigenetic changes in depth.
Clinical studies
Several clinical studies have examined an association between DNA methylation and ELS in the adolescent population. Cecil et al. [123] collected buccal epithelial cell DNA from 124 adolescents and youth—68% of whom reported experiencing some form of maltreatment during early life and investigated the array-based genome-wide methylation. The strongest association between methylomic variations and ELS was noted for physical abuse. Many identified loci were annotated to genes previously implicated in stress-related outcomes. For example, there was a significant association between physical abuse and presenilin 2 (PSEN2), sexual abuse and glutamate ionotropic receptor NMDA type subunit 2D (GRIN2D), and physical neglect and synaptojanin 2 (SYNJ2). Gene ontology analyses revealed that different types of ELS not only showed unique methylation patterns enriched for specific biological processes but also shared a common epigenetic signature, primarily related to neural development and organismal growth. The data suggest that epigenetic changes can distinguish the type of ELS and psychopathology.
Since epigenetic modifications are tissue-specific, Nieratschker et al. [124] applied a unique cross-species and cross-tissue approach to examine ELS-induced epigenetic changes. They reported that several regions in MORC1 (MORC family CW-type zinc finger 1) were differentially methylated in response to ELS in CD34+ cells and CD+ T cells derived from the blood of human and monkey neonates, as well as in CD3+ T cells derived from the blood of adolescent monkeys and in the prefrontal cortex of adult rats. A gene-set analysis from a genome-wide association study also suggested an association of MORC1 with MDD. This study is the first to identify epigenetic marks on a gene present in blood cell progenitors at birth and in the brain in adulthood, which shows an association with depression.
In cord blood from newborns with ELS in the prenatal stage, Devlin et al. [125] found significantly decreased promoter DNA methylation of the serotonin transporter gene (SLC6A4). NR3C1 DNA methylation from cord blood was also associated with newborns with ELS [126, 127]. Unternaehrer et al. [128] examined whether maternal adversities and cortisol levels during pregnancy predict cord blood DNA methylation of the oxytocin receptor (OXTR) and found that OXTR methylation was significantly associated with newborns with ELS in the prenatal stage. Interestingly, the number of stressful life events and maternal cortisol profile were negatively associated with OXTR DNA methylation. This suggests that distinct prenatal adversities predict decreased DNA methylation in the OXTR gene, which is relevant for childbirth, maternal behavior, and the well-being of mother and offspring.
Since childhood trauma affects social cognition and the basic processing of social cues, studies have examined whether epigenetic changes in the OXT gene contribute to long-term behavioral effects. Lesemann et al. [129] examined the N170 response to neutral faces in relation to participants’ recalled childhood trauma and methylation of oxytocin structural (OXTg) and oxytocin receptor (OXTRg) genes. They reported that OXTg and OXTRg methylation in female adolescents with ELS were associated with electroencephalographic N170 response to faces, which is a measure to capture neural response, and predicated a weakened N170 response in those with high methylation, and hyper-vigilance with participants with low methylation. Nishitani et al. [130] reported that DNA methylation-based age in adolescents with ELS was significantly increased compared with healthy controls using Pediatric-Buccal-Epigenetic (PedBE) clock methods, suggesting that PedBE age acceleration can be widely used as a biological marker for predicting atypical developmental features, including developmental vulnerabilities caused by maltreatment.
Sumner et al. [131] collected 113 saliva samples from adolescents (ages 8–16 years) with ELS reflecting the dimensions of threat and deprivation and applied the Illumina EPIC BeadChip array. Adjusting for lifetime experience of neglect, lifetime experience of abuse was associated with DNA methylation at 4 CpG sites (cg20241299, cg08671764, cg27152686, and cg24241897), whereas, adjusting for abuse, DNA methylation was associated with one CpG site (cg03135983). This study provides crucial evidence that DNA methylation changes over time with ongoing adverse experiences and that these experiences are characterized by distinct DNA methylation patterns. Grasso et al. [132] reported that FKBP5 DNA methylation in newborns with the T allele in SNPs was associated with maternal threat-related ELS, such as violence, but not with deprivation-related ELS, such as neglect. In an interesting study, Serpeloni et al. [133] examined the molecular mechanisms that mediate long-term consequences of early stress across generations. They determined the genome-wide DNA methylation profile in 121 children and tested for associations with exposure to grandmaternal interpersonal violence during pregnancy. They found methylation variations of five CpG sites significantly associated with the grandmother’s report of exposure to violence while pregnant with the mothers of the children, supporting the idea that DNA methylation may serve as a biological mechanism in the transmission of stress across generations.
Although many previous clinical studies have reported an association between DNA methylation and mental disorders in adult patients with ELS, to the best of our knowledge, there are only a few studies pertaining to DNA methylation and mental disorders in adolescents with ELS. Weder et al. [134] examined whether epigenetic markers can predict dimensional ratings of depression in maltreated children. Using a genome-wide methylation study, they found that the changes in DNA methylation of DNA-binding protein inhibitor ID-3 (ID3), glutamate receptor, ionotropic N-methyl-D-aspartate (NMDA) 1 (GRIN1), and tubulin polymerization promoting protein (TPPP) were predictors of depression in these children. DNA methylation in FKBP5 was also found to be associated with the rs1360780 SNP in bipolar disorder with ELS [135]. Yang et al. [136] collected saliva DNA samples from 96 adolescents with ELS and 96 controls and investigated the array-based genome-wide methylation. They revealed significant differences in DNA methylation at 2868 CpG sites between adolescents with ELS and controls. In blood samples from 46 adolescents with ELS, Radtke et al. [137] found that glucocorticoid receptor (hGR) gene methylation was associated with mental disorders in adolescents with ELS, especially cg1760381 methylation was associated with symptoms of borderline personality disorder. In an interesting study, Kaufman et al. [138] examined an association between DNA methylation and fMRI outcomes in 157 adolescent subjects with ELS. They reported that orthodenticle homeobox 2 (OTX2) methylation was associated with the right ventral medial prefrontal cortex and bilateral regions of the medial frontal cortex and cingulate cortex. Efstathopoulos et al. [139] collected saliva DNA from 1149 adolescents and revealed that exon 1 of NR3C1 methylation was significantly associated between depressive symptoms and ELS. In saliva samples from 247 adolescents with ELS, Sumner et al. [140] investigated methylation age using array-based genome-wide methylation. The threat-related ELS was uniquely associated with older DNA methylation age, but not deprivation-related ELS. Interestingly, depressive symptoms were associated with older DNA methylation age. This study suggests that early threat-related experiences may particularly be associated with accelerated biological aging in youths, which may be a mechanism linking ELA with depressive symptoms.
Serpeloni et al. [141] studied psychiatric illnesses and genome-wide DNA methylation following intimate partner violence (IPV) during pregnancy. They found that mothers and children with IPV had elevated depression, PTSD, and anxiety symptoms. Surprisingly, when IPV occurred during and after pregnancy, these problems were absent in children. They revealed that following prenatal IPV, DNA methylation in NR3C1 and FKBP5 genes were most methylated in adolescents with ELS. These children also showed more DNA methylation in heterochromatin-like regions, previously associated with stress/disease resilience. These results indicate an enhanced ability to terminate hormonal stress responses in prenatally stressed children and provide novel insights on how prenatal stress may epigenetically shape resilience in humans.
The studies mentioned above provide a glimpse into the use of various epigenetic approaches in examining ELS and associated psychiatric illnesses. Given the use of varied subject populations and methods to evaluate behavioral responses, it is challenging to assess uniformity in the reported findings; nevertheless, these studies clearly show that DNA methylation plays a crucial role in ELS-induced behavioral changes. A large number of studies have focused on FKBP5 and NR3C1 and OXT gene, broadly associated with stress social and emotional behavior, respectively, and found a consistent response to ELS and depression. Similar findings have been reported in the adult population [142]. Whether these effects are broadly associated with ELS and depression, regardless of age, needs further study.
MicroRNAs as mediators of ELS and depression
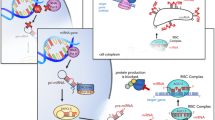
MicroRNAs (miRNAs) are a class of single-stranded small non-coding RNAs that act as an important epigenetic modifier and regulate protein levels of target messenger RNAs (mRNAs). They play vital roles in a variety of developmental processes, including cell proliferation, differentiation, synaptogenesis, synaptic plasticity, and apoptosis [143, 144]. MiRNAs are generated from short hairpin RNAs by two ribonuclease III-type proteins and, as the name indicates, are short in length (~22 nucleotides) [145]. By altering the intracellular stability of mRNAs post-transcriptionally, miRNAs regulate protein-coding genes and, subsequently, protein production [6, 97]. MiRNAs are synthesized in the nucleus where primary miRNA (pri-miRNA) with a hairpin loop structure is produced from the transcription of the miRNA gene by RNA polymerase II. Pri-miRNA is cleaved into precursor miRNA (pre-miRNA) by Drosha ribonuclease III (DROSHA) and Di George syndrome critical region in gene 8 (DGCR8). Pre-miRNA is then transported from the nucleus to the cytoplasm by Exportin 5 (EXPO-5), where pre-miRNA is converted into miRNA duplex by Dicer, an endonuclease cytoplasmic RNase III enzyme, and trans activation response RNA-binding protein (TRBP). MiRNA duplex is loaded onto an Argonaute (AGO) protein where only one strand is selected by the AGO protein as the mature miRNA. The mature miRNA with AGO protein forms the RNA-induced silencing complex (RISC). Post-transcriptional modifications of coding genes mediated by miRNAs generally happen through RISC, which induces target mRNA degradation or translational repression [145]. MiRNA binding sites are located in the 3′ untranslated region (UTR) of target mRNAs which is determined by their 5′ end from 2 to 7 nucleotide position as miRNA seed [145, 146].
In the past few years, a considerable amount of research has been conducted to examine the role of miRNAs in neuropsychiatric disease [147,148,149,150,151,152,153,154,155,156]. We and other investigators have examined the expression of miRNAs in human postmortem brains of depressed subjects, in the brain of animals showing depression-like behavior, and in peripheral tissues such as blood, urine, and saliva [6, 96, 144, 150, 157,158,159,160,161,162,163,164,165,166,167]. We have demonstrated that miRNAs form highly correlated networks in the brains of MDD subjects that differ from healthy controls, suggesting that miRNA networks can give rise to specific behavioral phenotypes [96, 168, 169]. We have also shown that rats who display hopelessness (i.e., learned helplessness), a clinical phenotype of suicide risk, has a blunted frontal cortical miRNA response to acute stress compared to non-hopeless rats [170], suggesting that aberrant miRNA expression can lead to deficits in the coping response to stress. Genetic differences in miRNA expression can also influence the coping response to a stressor. The stress-sensitive F344 rats that have an exaggerated release of corticosterone (CORT) to stressors show increased expression of hypothalamic miR-18a that binds to 3’UTR of glucocorticoid receptor and reduces its expression [171]. This results in increased CORT release by reduced feedback regulation. In addition, exposure of neurons to excessive CORT results in a decrease in the BDNF-dependent expression of postsynaptic proteins via suppression of miR-132 [172]. MiRNAs dynamically fluctuate, temporally and spatially, throughout the lifespan of an organism. For example, two studies focusing on Lin28/let-7 system in rodents and monkeys found that the expression of Lin28a and Lin28b from neonatal/juvenile to adult ages were significantly different compared with adults [173, 174]. Let-7b had the largest increase between puberty and adulthood in rats, increasing by 400%. Significant expression changes in miR-9, miR-132, and miR-145 were also observed between neonatal to adult ages.
MiRNAs are also released into the blood and CSF [175,176,177]. Circulatory miRNAs are stably expressed under healthy conditions, but the miRNA profile changes dramatically under pathological states, suggesting that peripheral miRNAs can be used as disease biomarkers [178, 179], including psychiatric illnesses [164, 180,181,182,183].
ELS Studies of miRNAs
Animal studies
A detailed review of literature has recently been published by us, highlighting the role of miRNAs in ELS [6]. More recently, our group used MS as a rodent model of ELS and tested whether miRNAs target serotonin genes to regulate ELS-induced depression-like behavior and whether this effect is sex-dependent. We also examined whether environmental enrichment prevents susceptibility to depression- and anxiety-like behavior following MS and whether enrichment effects are mediated through serotonin genes and their corresponding miRNAs [184]. It was observed that MS decreased sucrose preference, which was reversed by enrichment. Males also exhibited greater changes in forced swim climbing and escape latency tests only following enrichment. Expression levels of serotonin transporter Slc6a4 and 5-hydroxytryptamine receptor 1A (Htr1a) were upregulated in the frontal cortex following MS. In male MS rats, enrichment reversed Htr1a expression to levels similar to control rats. MiR-200a-3p and miR-322-5p, which target SLC6A4, were decreased by MS. An HTR1A-targeting miRNA, miR-320-5p, was also downregulated by MS and showed slight reversal by enrichment in male animals. MiR-320-5p targeting of Htr1a was validated in vitro using SHSY neuroblastoma cell lines. Altogether, this study implicates miRNA interaction with the serotonin pathway in ELS-induced susceptibility to depression-related reward deficits. Furthermore, because of its recovery by enrichment in males, miR-320 may represent a viable sex-specific target for reward-related deficits in major depressive disorder.
Our group also tested the expression of miRNAs in the hypothalamus following ELS and susceptibility to depression-like behavior and whether sex or acute stress exacerbates this response [185]. We further tested whether environmental enrichment promotes early life and adult behavioral stress resilience and its effect on hypothalamic miRNA and gene expression. Interaction of MS, enrichment, restraint stress, and sex showed alterations in miRs-29, -124, -132, -144, and -504. Sex had a significant effect on a large number of miRNAs. Also, environment enrichment reversed the downregulation of miR-29b-1-5p and -301b-3p in MS. qPCR analysis showed that MAPK6 and MMP19, targets of miR-301b-3p, were upregulated in MS and reversed by enrichment. Additionally, miR-219a was hypermethylated in MS, coinciding with decreased miR-219a expression. This study suggests that sex plays a critical role in the hypothalamic miRNA response to both ELS and acute stress, with males expressing greater changes following postnatal stress. Moreover, enrichment significantly altered behavior as well as hypothalamic miRNA expression and their gene targets.
Using unpredictable chronic mild stress in adolescence as an ELS model, Guo et al. [186] investigated the effects of miR-15b using C57 GAD67-GFP mice. They found that the injection of miR-15b antagomir significantly improved depression-like behavior and improved the reductions of excitatory synapse and syntaxin-binding protein 3 (STXBP3A)/vesicle-associated protein 1 (VAMP1) expressions in ELS model. The injection of a miR-15b analog into the nucleus accumbens induced similar behavior and down-regulation of STXBP3A/VAMP1 expressions in ELS mice.
Clinical studies
Clinical studies of miRNAs in ELS are quite limited. In patients with bipolar disorder and history of ELS, Prados et al. [187] reported that hyper-methylation miR-124 promoter was correlated with ELS history and symptom severity compared to depressed patients with no ELS history. In a sample of 32 controls (11 with and 22 without an early trauma history), Cattane et al. [188] reported differential regulation in 80 miRNAs in the ELS group compared with participants without ELS. MiR-29b-3p, miR-29c-3p, and miR-16-5p were significantly upregulated, while miR-200b-5p and miR-125b-1-3p were significantly downregulated. In prenatally stressed rodents, these authors found a decrease only in miR-125-1-3p, suggesting that miR-125-1-3p may be specifically responsive to ELS, and the effects are lasting and consistent across species. In another study, Suderman et al. [189] reported analyzed the methylation of 489 miRNAs using methylated DNA immunoprecipitation and revealed that an altered pattern of methylation in 39 miRNAs was associated with ELS. Confirmatory analysis showed that miR-514, let-7d, miR-520c, miR-215, miR-519a, and miR-519e were hypermethylated, whereas miR-203 was hypomethylated. So far, there are only a few clinical studies examining the relationship between miRNA and mental disorders in adolescents with ELS. Ran et al. [161] collected miRNAs from serum extracellular vesicles and did genome-wide miRNA sequencing in a sample of 17 adolescents (9 untreated MDD subjects and 8 controls) and validated it in a sample of 72 adolescents (34 untreated MDD subjects and 38 controls). They identified 18 upregulated miRNA and 14 downregulated miRNAs in MDD subjects when compared with controls and revealed that the expressions of miR-450a-2-3p, miR-556-3p, and miR-2115-3 were significantly different, and there was an association between miR-450a-2-3p and ELS.
MiRNAs in the field of adolescent depression are an emerging area of research. As described above, both animal and human studies indicate that several miRNAs are responsive to ELS. Whether these miRNAs can serve as vulnerability factors in the development of depression associated with ELS is not clearly known; however, as shown by us [185], the expression of miRNAs is significantly altered in adult MS rats who showed depression phenotype, and these changes are sex-specific. Further studies will be required to examine the impact of miRNAs on ELS and their susceptibility to developing depression during adolescence. Also, comparing adult and adolescent subjects with depression who had prior experience with trauma will determine if miRNA changes are similar across age groups.
Conclusions and future directions
A summary of findings pertaining to ELS and its impact on DNA methylation and miRNAs is provided in Tables 1 and 2. As can be seen, whereas there have been several studies on the effect of ELS on epigenetic modifications in adulthood, only a handful of studies are available showing such effects in adolescents, especially in human subjects. Also, there are only a few studies that have examined associations between the frequency or intensity of ELS, epigenetic changes, and subsequent onset of adolescent depression. In addition, there is no large-scale study in depressed patients who had gone through other stresses from the time of the ELS to the time when they were studied. Thus, it is difficult to dissect an association between the initial effects of ELS on epigenetic changes and those that occur over a period of time during adolescent depression. Further clinical longitudinal studies of the impact of each patient’s life events, including ELS, on epigenetic changes, should be studied.
Besides these limitations, several outstanding questions need answers: (1) Are there commonalities in ELS-associated methylation marks between adults and adolescents? (2) Do epigenetic changes affect mental and physical health in youth and beyond? (3) Are the changes sex-specific? (4) Are DNA methylation patterns heritable, and whether this can explain part of the heritability of depression? (5) Do different types of early life stresses have differential impacts on epigenetic programming, and are there shared vs. unique epigenetic signatures of maltreatment types? (6) Can these epigenetic signatures be used as predictors of different psychiatric illnesses? (7) Do prenatal and postnatal stresses commonly target epigenetic programming to impact mental health and behavior? (8) Can the epigenetic modifiers be used to reverse the clinical outcome? It is interesting to note that contrary to the finding in adults, Devlin et al. [125] reported no significant effect on BDNF methylation in adolescents with ELS, whereas Dukal et al. [190] reported that SLC6A4 methylation in females was significantly higher than in males, but it was not associated with ELS. On the other hand, Mckibben et al. [185] reported sex-specific changes in ELS-induced depression in several miRNAs. Using array-based genome-wide methylation, Wikenius et al. [191] found no significant associations between maternal depressive symptoms and DNA methylation in babies at 6 weeks and 12 months. Not related to ELS, Cardenas et al. [192] found that maternal use of antidepressants was associated with Zinc Finger Protein 575 gene (ZNF575) methylation in newborns but not in early childhood. Thus, the associations between ELS and epigenetic changes are quite complicated. Further studies will be needed to test the role of potential moderators in the identified associations, including the age of onset and chronicity of maltreatment exposure.
Animal studies have shown that the effects of caregiver deprivation on hypothalamic-pituitary-adrenal (HPA) axis function are dependent on the timing of exposure [193]. Animals separated on the third postnatal day demonstrate no immediate alterations in HPA function, whereas HPA responsiveness is markedly elevated in those separated on PND11. Additionally, examination of long-term alterations in stress reactivity indicated that animals separated on PND3 and PND11 showed hyper and hypo HPA responsiveness in adulthood [194]. These studies suggest that the timing of exposure to early-life trauma is critical in stress responsiveness. Whether such separation can be studied in humans is a matter of debate, given that most children exposed to early-life adversity continue to be exposed to adverse conditions throughout development.
In terms of treatment, Levine et al. [195] reported that histone deacetylases (HDACs) 1, 3, 7, 8, and 10 mRNA expressions were significantly decreased in the Balb/c mice ELS model and fluoxetine augmentated all histone modifications. Elmer et al. [196] reported that ketamine metabolite (2R,6R)-hydroxynorketamine reversed behavioral despair produced by adolescent trauma. Furthermore, recently, Wrobel et al. [197] examined the role of childhood trauma in treating bipolar disorder. The effect of childhood trauma on treatment outcomes was evaluated among participants randomized to treatment with lithium or quetiapine. It was found that although participants with a history of any childhood trauma presented with greater symptom severity and functional impairment at most study visits, participants with and without a history of any childhood trauma showed similar rates of improvement in symptom severity and functional impairment over the 24 weeks of treatment.
In conclusion, although several studies show that epigenetic modifications are critical in mediating the effect of ELS on various aspects of behavior in adulthood; however, a large body of work is needed to dissect the impact of ELS on mental disorders in adolescents. More recently, epigenetic contributions to the intergenerational and transgenerational heritability of mental disorders are actively being pursued [198,199,200,201,202,203]. This includes DNA modifications and non-coding RNAs, both contributing to brain circuitry changes and, ultimately, behavior. Depressive states in mothers have also been shown to be associated with their children’s functioning, including potential vulnerabilities to the onset of depression in their later lives [200]. ELS also mediates intergenerational and transgenerational transmission of epigenetic changes [198, 204], and can cause vulnerability to develop psychiatric diseases [205]. This area of research is of high significance and may shed light on the mechanisms of ELS-mediated development of depression and suicidal behavior in adolescents.
Epigenetics has emerged as an important potential target of treatment development. It remains to be seen if epigenetic changes will emerge as bona fide treatment targets. However, the development of molecular approaches that target specific epigenetic marks may ultimately treat specific environmentally-induced diseases. However, this approach may also improve a broad set of disorders that are affected by similar environmental antecedents. The epigenetic changes may serve as common and possibly modifiable vulnerability factors for trauma-related disorders more broadly. Targeting these changes might lead to novel treatments and, more importantly, ways of reversing the effects of ELS and thereby reducing the risk for a range of mental disorders. This could also bring a new generation of risk-modifying approaches that may provide ways of preventing and not just treating diseases.
References
Teicher MH. Childhood trauma and the enduring consequences of forcibly separating children from parents at the United States border. BMC Med. 2018;16:146.
Rivara F, Adhia A, Lyons V, Massey A, Mills B, Morgan E, et al. The effects of violence on health. Health Aff. 2019;38:1622–9.
Wade R, Cronholm PF, Fein JA, Forke CM, Davis MB, Harkins-Schwarz M, et al. Household and community-level Adverse Childhood Experiences and adult health outcomes in a diverse urban population. Child Abus Negl. 2016;52:135–45.
Campbell JA, Walker RJ, Egede LE. Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. Am J Prev Med. 2016;50:344–52.
Ports KA, Ford DC, Merrick MT. Adverse childhood experiences and sexual victimization in adulthood. Child Abus Negl. 2016;51:313–22.
Allen L, Dwivedi Y. MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Mol Psychiatry. 2020;25:308–20.
Widom CS, Czaja SJ, Bentley T, Johnson MS. A prospective investigation of physical health outcomes in abused and neglected children: new findings from a 30-year follow-up. Am J Public Health. 2012;102:1135–44.
Huang H, Yan P, Shan Z, Chen S, Li M, Luo C, et al. Adverse childhood experiences and risk of type 2 diabetes: a systematic review and meta-analysis. Metabolism. 2015;64:1408–18.
Shah S, Mackinnon A, Galletly C, Carr V, McGrath JJ, Stain HJ, et al. Prevalence and impact of childhood abuse in people with a psychotic illness. Data from the second Australian national survey of psychosis. Schizophr Res. 2014;159:20–26.
Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA. 2001;286:3089–96.
Ferraz L, Portella MJ, Vállez M, Gutiérrez F, Martín-Blanco A, Martín-Santos R, et al. Hostility and childhood sexual abuse as predictors of suicidal behaviour in borderline personality disorder. Psychiatry Res. 2013;210:980–5.
Fuller-Thomson E, Baird SL, Dhrodia R, Brennenstuhl S. The association between adverse childhood experiences (ACEs) and suicide attempts in a population‐based study. Child Care Heal Dev. 2016;42:725–34.
Liang H, Olsen J, Yuan W, Cnattingus S, Vestergaard M, Obel C, et al. Early life bereavement and schizophrenia. Medicine. 2016;95:e2434.
Merrick MT, Ports KA, Ford DC, Afifi TO, Gershoff ET, Grogan-Kaylor A. Unpacking the impact of adverse childhood experiences on adult mental health. Child Abus Negl. 2017;69:10–19.
de Bach SL, Molina MAL, Jansen K, da Silva RA, de Souza LDM. Suicide risk and childhood trauma in individuals diagnosed with posttraumatic stress disorder. Trends Psychiatry Psychother. 2018;40:253–7.
Obikane E, Shinozaki T, Takagi D, Kawakami N. Impact of childhood abuse on suicide-related behavior: Analysis using marginal structural models. J Affect Disord. 2018;234:224–30.
Alberdi-Paramo I, Saiz-Gonzalez MD, Diaz-Marsa M, Carrasco-Perera JL. Bullying and childhood trauma events as predictive factors of suicidal behavior in borderline personality disorder: preliminary findings. Psychiatry Res. 2019;285:112730.
Thompson MP, Kingree JB, Lamis D. Associations of adverse childhood experiences and suicidal behaviors in adulthood in a U.S. nationally representative sample. Child Care Heal Dev. 2019;45:121–8.
Ng QX, Yong BZJ, Ho CYX, Lim DY, Yeo W-S. Early life sexual abuse is associated with increased suicide attempts: An update meta-analysis. J Psychiatr Res. 2018;99:129–41.
Santo FD, Carballo JJ, Velasco A, Jiménez-Treviño L, Rodríguez-Revuelta J, Martínez-Cao C, et al. The Mediating role of impulsivity in the relationship between suicidal behavior and early traumatic experiences in depressed subjects. Front Psychiatry. 2020;11:538172.
Prokopez CR, Vallejos M, Farinola R, Alberio G, Caporusso GB, Cozzarin LG, et al. The history of multiple adverse childhood experiences in patients with schizophrenia is associated with more severe symptomatology and suicidal behavior with gender-specific characteristics. Psychiatry Res. 2020;293:113411.
O’Neill S, O’Connor RC. Suicide in Northern Ireland: epidemiology, risk factors, and prevention. Lancet Psychiatry. 2020;7:538–46.
Gloger S, Martínez P, Behn A, Chacón MV, Cottin M, de Medina DD, et al. Population-attributable risk of adverse childhood experiences for high suicide risk, psychiatric admissions, and recurrent depression, in depressed outpatients. Eur J Psychotraumatol. 2021;12:1874600.
Laroche DG, Godin O, Belzeaux R, M’Bailara K, Loftus J, Courtet P, et al. Association between childhood maltreatment and the clinical course of bipolar disorders: a survival analysis of mood recurrences. Acta Psychiat Scand. 2022;145:373–83.
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58.
Kappel RH, Livingston MD, Patel SN, Villaveces A, Massetti GM. Prevalence of adverse childhood experiences (ACEs) and associated health risks and risk behaviors among young women and men in Honduras. Child Abus Negl. 2021;115:104993.
Johnson RM, Hill AV, Jones VC, Powell TW, Dean LT, Gilreath TD. Racial/ethnic inequities in adverse childhood experiences and selected health-related behaviors and problems among Maryland adolescents. Health Promot Pract. 2021:15248399211008238.
Houtepen LC, Heron J, Suderman MJ, Fraser A, Chittleborough CR, Howe LD. Associations of adverse childhood experiences with educational attainment and adolescent health and the role of family and socioeconomic factors: A prospective cohort study in the U.K. Plos Med. 2020;17:e1003031.
Lee M-C, Huang N, Chen C-Y. Effects of childhood adversity trajectories on mental health outcomes in late adolescence: The buffering role of parenting practices in Taiwan. Child Abus Negl. 2020;109:104705.
Cohrdes C, Mauz E. Self-efficacy and emotional stability buffer negative effects of adverse childhood experiences on young adult health-related quality of life. J Adolesc Health. 2020;67:93–100.
Karatekin C. Adverse childhood experiences (ACEs), Stress and mental health in college students. Stress Health. 2018;34:36–45.
Thompson R, Flaherty EG, English DJ, Litrownik AJ, Dubowitz H, Kotch JB, et al. Trajectories of adverse childhood experiences and self-reported health at age 18. Acad Pediatr. 2015;15:503–9.
Anto M, Jaffee S, Tietjen G, Mendizabal A, Szperka C. Adverse childhood experiences and frequent headache by adolescent self-report. Pediatr Neurol. 2021;121:51–55.
Rosa MJ, Lee AG, Wright RJ. Evidence establishing a link between prenatal and early-life stress and asthma development. Curr Opin Allergy Clin Immunol. 2018;18:148–58.
Lensch T, Clements-Nolle K, Oman RF, Evans WP, Lu M, Yang W. Adverse childhood experiences and suicidal behaviors among youth: The buffering influence of family communication and school connectedness. J Adolesc Health. 2021;68:945–52.
Polanco-Roman L, Alvarez K, Corbeil T, Scorza P, Wall M, Gould MS, et al. Association of childhood adversities with suicide ideation and attempts in Puerto Rican young adults. JAMA Psychiatry. 2021;78:896–902.
Clements-Nolle K, Lensch T, Yang Y, Martin H, Peek J, Yang W. Attempted suicide among adolescents in military families: The mediating role of adverse childhood experiences. J Interpers Violence. 2021;36:11743–54.
Meza JI, Owens EB, Hinshaw SP. Childhood predictors and moderators of lifetime risk of self-harm in girls with and without attention-deficit/hyperactivity disorder. Dev Psychopathol. 2021;33:1351–67.
Törnblom AW, Sorjonen K, Runeson B, Rydelius P-A. Life events and coping strategies among young people who died by suicide or sudden violent death. Front Psychiatry. 2021;12:670246.
Li S, Wang S, Gao X, Jiang Z, Xu H, Zhang S, et al. Patterns of adverse childhood experiences and suicidal behaviors in adolescents: A four-province study in China. J Affect Disord. 2021;285:69–76.
Geselowitz B, Whalen DJ, Tillman R, Barch DM, Luby JL, Vogel A. Preschool age predictors of adolescent borderline personality symptoms. J Am Acad Child Adolesc Psychiatry. 2021;60:612–22.
McMahon EM, Corcoran P, Keeley H, Clarke M, Coughlan H, Wasserman D, et al. Risk and protective factors for psychotic experiences in adolescence: a population-based study. Psychol Med. 2021;51:1220–8.
Aytur SA, Carlino S, Bernard F, West K, Dobrzycki V, Malik R. Social‐ecological theory, substance misuse, adverse childhood experiences, and adolescent suicidal ideation: applications for community-academic partnerships. J Community Psychol. 2022;50:265–84.
Kim B-KE, Gilman AB, Thompson N, Leon JD. Statewide trends of trauma history, suicidality, and mental health among youth entering the juvenile justice system. J Adolesc Health. 2021;68:300–7.
Jia Z, Wen X, Chen F, Zhu H, Li C, Lin Y, et al. Cumulative exposure to adverse childhood experience: Depressive symptoms, suicide intensions and suicide plans among senior high school students in Nanchang city of China. Int J Environ Res Public Health. 2020;17:4718.
Thai TT, Cao PLT, Kim LX, Tran DP, Bui MB, Bui HHT. The effect of adverse childhood experiences on depression, psychological distress and suicidal thought in Vietnamese adolescents: Findings from multiple cross-sectional studies. Asian J Psychiatry. 2020;53:102134.
Björkenstam E, Hjern A, Björkenstam C, Kosidou K. Association of cumulative childhood adversity and adolescent violent offending with suicide in early adulthood. JAMA Psychiatry. 2018;75:185–93.
Cluver L, Orkin M, Boyes ME, Sherr L. Child and adolescent suicide attempts, suicidal behavior, and adverse childhood experiences in South Africa: a prospective study. J Adolesc Health. 2015;57:52–59.
Stewart JG, Kim JC, Esposito EC, Gold J, Nock MK, Auerbach RP. Predicting suicide attempts in depressed adolescents: clarifying the role of disinhibition and childhood sexual abuse. J Affect Disord. 2015;187:27–34.
Infurna MR, Reichl C, Parzer P, Schimmenti A, Bifulco A, Kaess M. Associations between depression and specific childhood experiences of abuse and neglect: a meta-analysis. J Affect Disord. 2016;190:47–55.
LeMoult J, Humphreys KL, Tracy A, Hoffmeister J-A, Ip E, Gotlib IH. Meta-analysis: exposure to early life stress and risk for depression in childhood and adolescence. J Am Acad Child Adolesc Psychiatry. 2020;59:842–55.
Grigsby TJ, Rogers CJ, Albers LD, Benjamin SM, Lust K, Eisenberg ME, et al. Adverse childhood experiences and health indicators in a young adult, college student sample: differences by gender. Int J Behav Med. 2020;27:660–7.
Serafini G, Pompili M, Innamorati M, Gentile G, Borro M, Lamis DA, et al. Gene variants with suicidal risk in a sample of subjects with chronic migraine and affective temperamental dysregulation. Eur Rev Med Pharm Sci. 2012;16:1389–98.
Enns MW, Cox BJ, Afifi TO, Graaf RD, Have MT, Sareen J. Childhood adversities and risk for suicidal ideation and attempts: a longitudinal population-based study. Psychol Med. 2006;36:1769–78.
Ford JD, Gómez JM. Self-injury and suicidality: the impact of trauma and dissociation. J Trauma Dissociation. 2015;16:225–31.
Bruffaerts R, Demyttenaere K, Borges G, Haro JM, Chiu WT, Hwang I, et al. Childhood adversities as risk factors for onset and persistence of suicidal behaviour. Br J Psychiatry. 2010;197:20–27.
Stansfeld SA, Clark C, Smuk M, Power C, Davidson T, Rodgers B. Childhood adversity and midlife suicidal ideation. Psychol Med. 2017;47:327–40.
Rod NH, Bengtsson J, Budtz-Jørgensen E, Clipet-Jensen C, Taylor-Robinson D, Andersen A-MN, et al. Trajectories of childhood adversity and mortality in early adulthood: a population-based cohort study. Lancet. 2020;396:489–97.
Davidson JR, Hughes DC, George LK, Blazer DG. The association of sexual assault and attempted suicide within the community. Arch Gen Psychiatry. 1996;53:550–5.
Molnar BE, Berkman LF, Buka SL. Psychopathology, childhood sexual abuse and other childhood adversities: relative links to subsequent suicidal behaviour in the US. Psychol Med. 2001;31:965–77.
Afifi TO, Enns MW, Cox BJ, Asmundson GJG, Stein MB, Sareen J. Population attributable fractions of psychiatric disorders and suicide ideation and attempts associated with adverse childhood experiences. Am J Public Health. 2008;98:946–52.
Roy A. Characteristics of cocaine-dependent patients who attempt suicide. Am J Psychiatry. 2001;158:1215–9.
Roy A. Childhood trauma and attempted suicide in alcoholics. J Nerv Ment Dis. 2001;189:120–1.
Sarchiapone M, Carli V, Cuomo C, Roy A. Childhood trauma and suicide attempts in patients with unipolar depression. Depress Anxiety. 2007;24:268–72.
Brodsky BS, Stanley B. Adverse childhood experiences and suicidal behavior. Psychiatr Clin N Am. 2008;31:223–35.
Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170:1114–33.
Zatti C, Rosa V, Barros A, Valdivia L, Calegaro VC, Freitas LH, et al. Childhood trauma and suicide attempt: a meta-analysis of longitudinal studies from the last decade. Psychiatry Res. 2017;256:353–8.
Carbone JT, Jackson DB, Holzer KJ, Vaughn MG. Childhood adversity, suicidality, and non-suicidal self-injury among children and adolescents admitted to emergency departments. Ann Epidemiol. 2021;60:21–27.
Wang S, Xu H, Li S, Jiang Z, Wan Y. Sex differences in the determinants of suicide attempt among adolescents in China. Asian J Psychiatr. 2020;49:101961.
Roy B, Dwivedi Y. Modeling endophenotypes of suicidal behavior in animals. Neurosci Biobehav Rev. 2021;128:819–27.
Roy B, Dwivedi Y. Understanding epigenetic architecture of suicide neurobiology: a critical perspective. Neurosci Biobehav Rev. 2017;72:10–27.
Dwivedi Y. Involvement of brain-derived neurotrophic factor in late-life depression. Am J Geriatr Psychiatry. 2013;21:433–49.
Simsek S, Uysal C, Kaplan I, Yuksel T, Aktas H. BDNF and cortisol levels in children with or without posttraumatic stress disorder after sustaining sexual abuse. Psychoneuroendocrinology. 2015;56:45–51.
Zhao M, Chen L, Yang J, Han D, Fang D, Qiu X, et al. BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. J Affect Disord. 2018;227:226–35.
Chen J, Li X, McGue M. The interacting effect of the BDNF Val66Met polymorphism and stressful life events on adolescent depression is not an artifact of gene-environment correlation: evidence from a longitudinal twin study. J Child Psychol Psychiatry. 2013;54:1066–73.
Carver CS, Johnson SL, Joormann J, LeMoult J, Cuccaro ML. Childhood adversity interacts separately with 5-HTTLPR and BDNF to predict lifetime depression diagnosis. J Affect Disord. 2011;132:89–93.
Rimay T, Benak I, Kiss E, Baji I, Feher A, Juhasz A, et al. BDNF Val66Met polymorphism and stressful life events in melancholic childhood-onset depression. Psychiatr Genet. 2015;25:249–55.
Kaufman J, Yang B-Z, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, et al. Brain-derived neurotrophic Factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–80.
Zhang L, Li Z, Chen J, Li X, Zhang J, Belsky J. The BDNF Val66Met polymorphism interacts with maternal parenting influencing adolescent depressive symptoms: Evidence of differential susceptibility model. J Youth Adolesc. 2016;45:471–83.
Fan M, Li RH, Hu MS, Xiao LY, Zhou XD, Ran MS, et al. Association of Val66Met polymorphism at brain derived neurotrophic factor gene with depression among Chinese adolescents after Wenchuan earthquake: An 18months longitudinal study. Physiol Behav. 2017;179:16–22.
Comasco E, Åslund C, Oreland L, Nilsson KW. Three-way interaction effect of 5-HTTLPR, BDNF Val66Met, and childhood adversity on depression: a replication study. Eur Neuropsychopharmacol. 2013;23:1300–6.
Cruz-Fuentes CS, Benjet C, Martínez-Levy GA, Pérez-Molina A, Briones-Velasco M, Suárez-González J. BDNF Met66 modulates the cumulative effect of psychosocial childhood adversities on major depression in adolescents. Brain Behav. 2014;4:290–7.
Perea CS, Paternina AC, Gomez Y, Lattig MC. Negative affectivity moderated by BDNF and stress response. J Affect Disord. 2012;136:767–74.
Chen J, Li X, McGue M. Interacting effect of BDNF Val66Met polymorphism and stressful life events on adolescent depression. Genes Brain Behav. 2012;11:958–65.
Greca AML, Lai BS, Joormann J, Auslander BB, Short MA. Children’s risk and resilience following a natural disaster: Genetic vulnerability, posttraumatic stress, and depression. J Affect Disord. 2013;151:860–7.
Agnafors S, Comasco E, Bladh M, Sydsjö G, DeKeyser L, Oreland L, et al. Effect of gene, environment and maternal depressive symptoms on pre-adolescence behavior problems - a longitudinal study. Child Adolesc Psychiatry Ment Health. 2013;7:10.
Valderrama J, Miranda R. Early life stress predicts negative urgency through brooding, depending on 5-HTTLPR genotype: A pilot study with 6-month follow-up examining suicide ideation. Psychiatry Res. 2017;258:481–7.
Åslund C, Leppert J, Comasco E, Nordquist N, Oreland L, Nilsson KW. Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behav Genet. 2009;39:524–31.
Bortoluzzi A, Salum GA, Blaya C, Silveira PP, Grassi-Oliveira R, da Rosa ED, et al. Mineralocorticoid receptor genotype moderates the association between physical neglect and serum BDNF. J Psychiatr Res. 2014;59:8–13.
Isaksson J, Comasco E, Åslund C, Rehn M, Tuvblad C, Andershed H, et al. Associations between the FKBP5 haplotype, exposure to violence and anxiety in females. Psychoneuroendocrinology. 2016;72:196–204.
Wang Q, Shelton RC, Dwivedi Y. Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and posttraumatic stress disorder: a systematic review and meta-analysis. J Affect Disord. 2018;225:422–8.
Mistry S, Harrison JR, Smith DJ, Escott-Price V, Zammit S. The use of polygenic risk scores to identify phenotypes associated with genetic risk of bipolar disorder and depression: a systematic review. J Affect Disord. 2018;234:148–55.
Nelemans SA, Boks M, Lin B, Oldehinkel T, van Lier P, Branje S, et al. Polygenic risk for major depression interacts with parental criticism in predicting adolescent depressive symptom development. J Youth Adolesc. 2021;50:159–76.
Yoshino Y, Dwivedi Y. Non-coding RNAs in psychiatric disorders and suicidal behavior. Front Psychiatry. 2020;11:543893.
Roy B, Shelton RC, Dwivedi Y. DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation. J Psychiatr Res. 2017;89:115–24.
Roy B, Wang Q, Palkovits M, Faludi G, Dwivedi Y. Altered miRNA expression network in locus coeruleus of depressed suicide subjects. Sci Rep. 2017;7:4387.
Belzeaux R, Lin R, Ju C, Chay M-A, Fiori LM, Lutz P-E, et al. Transcriptomic and epigenomic biomarkers of antidepressant response. J Affect Disord. 2018;233:36–44.
Misra P, Liu S, Meng X. What DNA methylation modifications and/or genetic variations interact with childhood maltreatment in the development of depression: a systematic review. J Affect Disord. 2019;252:325–33.
Stenz L, Schechter DS, Serpa SR, Paoloni-Giacobino A. Intergenerational transmission of DNA methylation signatures associated with early life stress. Curr Genomics. 2018;19:665–75.
Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, et al. Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrinology. 2018;92:123–34.
Burns SB, Szyszkowicz JK, Luheshi GN, Lutz P-E, Turecki G. Plasticity of the epigenome during early-life stress. Semin Cell Dev Biol. 2018;77:115–32.
Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharm Toxicol. 2013;53:59–87.
Dong E, Pandey SC. Prenatal stress induced chromatin remodeling and risk of psychopathology in adulthood. Int Rev Neurobiol. 2021;156:185–215.
McGowan PO, Szyf M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol Dis. 2010;39:66–72.
Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019;20:590–607.
Chiarella J, Schumann L, Pomares FB, Frodl T, Tozzi L, Nemoda Z, et al. DNA methylation differences in stress-related genes, functional connectivity and gray matter volume in depressed and healthy adolescents. J Affect Disord. 2020;271:160–8.
Raffetti E, Melas PA, Landgren AJ, Andersson F, Forsell Y, Lavebratt C, et al. DNA methylation of the glucocorticoid receptor gene predicts substance use in adolescence: longitudinal data from over 1000 young individuals. Transl Psychiatry. 2021;11:477.
Roberson-Nay R, Lapato DM, Wolen AR, Lancaster EE, Webb BT, Verhulst B, et al. An epigenome-wide association study of early-onset major depression in monozygotic twins. Transl Psychiatry. 2020;10:301.
Masrour FF, Peeri M, Azarbayjani MA, Hosseini M-J. Voluntary exercise during adolescence mitigated negative the effects of maternal separation stress on the depressive-like behaviors of adult male rats: Role of NMDA receptors. Neurochem Res. 2018;43:1067–74.
Lesse A, Rether K, Gröger N, Braun K, Bock J. Chronic postnatal stress induces depressive-like behavior in male mice and programs second-hit stress-induced gene expression patterns of OxtR and AvpR1a in adulthood. Mol Neurobiol. 2017;54:4813–9.
Ganguly P, Holland FH, Brenhouse HC. Functional uncoupling NMDAR NR2A subunit from PSD-95 in the prefrontal cortex: effects on behavioral dysfunction and parvalbumin loss after early-life stress. Neuropsychopharmacology. 2015;40:2666–75.
Jahng JW, Ryu V, Yoo SB, Noh SJ, Kim JY, Lee JH. Mesolimbic dopaminergic activity responding to acute stress is blunted in adolescent rats that experienced neonatal maternal separation. Neuroscience. 2010;171:144–52.
Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, Brenhouse HC. Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology. 2016;71:19–30.
Jaric I, Rocks D, Cham H, Herchek A, Kundakovic M. Sex and estrous cycle effects on anxiety- and depression-related phenotypes in a two-hit developmental stress model. Front Mol Neurosci. 2019;12:74.
Leussis MP, Freund N, Brenhouse HC, Thompson BS, Andersen SL. Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Dev Neurosci. 2012;34:210–7.
Liu Y, Wang Z, Zhang X, Li S, Wu W, Li X, et al. A sex-dependent delayed maturation of visual plasticity induced by adverse experiences in early childhood. Neurobiol Stress. 2020;13:100256.
Wang R, Wang W, Xu J, Liu D, Jiang H, Pan F. Dynamic effects of early adolescent stress on depressive-like behaviors and expression of cytokines and JMJD3 in the prefrontal cortex and hippocampus of rats. Front Psychiatry. 2018;9:471.
Deng J-H, Yan W, Han Y, Chen C, Meng S-Q, Sun C-Y, et al. Predictable chronic mild stress during adolescence promotes fear memory extinction in adulthood. Sci Rep. 2017;7:7857.
Yang X, Ewald ER, Huo Y, Tamashiro KL, Salvatori R, Sawa A, et al. Glucocorticoid-induced loss of DNA methylation in non-neuronal cells and potential involvement of DNMT1 in epigenetic regulation of Fkbp5. Biochem Biophys Res Commun. 2012;420:570–5.
Kronman H, Torres-Berrío A, Sidoli S, Issler O, Godino A, Ramakrishnan A, et al. Long-term behavioral and cell-type-specific molecular effects of early life stress are mediated by H3K79me2 dynamics in medium spiny neurons. Nat Neurosci. 2021;24:667–76.
Fitzgerald E, Sinton MC, Wernig-Zorc S, Morton NM, Holmes MC, Boardman JP, et al. Altered hypothalamic DNA methylation and stress-induced hyperactivity following early life stress. Epigenetics Chromatin. 2021;14:31.
Seo MK, Lee JG, Park SW. Early life stress induces age-dependent epigenetic changes in p11 gene expression in male mice. Sci Rep. 2021;11:10663.
Cecil CAM, Smith RG, Walton E, Mill J, McCrory EJ, Viding E. Epigenetic signatures of childhood abuse and neglect: Implications for psychiatric vulnerability. J Psychiatr Res. 2016;83:184–94.
Nieratschker V, Massart R, Gilles M, Luoni A, Suderman MJ, Krumm B, et al. MORC1 exhibits cross-species differential methylation in association with early life stress as well as genome-wide association with MDD. Transl Psychiatry. 2014;4:e429.
Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS ONE. 2010;5:e12201.
Mulligan C, D’Errico N, Stees J, Hughes D. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics. 2012;7:853–7.
Hompes T, Izzi B, Gellens E, Morreels M, Fieuws S, Pexsters A, et al. Investigating the influence of maternal cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. J Psychiatr Res. 2013;47:880–91.
Unternaehrer E, Bolten M, Nast I, Staehli S, Meyer AH, Dempster E, et al. Maternal adversities during pregnancy and cord blood oxytocin receptor (OXTR) DNA methylation. Soc Cogn Affect Neurosci. 2016;11:1460–70.
Lesemann FHP, Spencer H, Montoya ER, Kraaijenvanger EJ, He Y, Branje S, et al. Methylation of oxytocin related genes and early life trauma together shape the N170 response to human faces. Eur Neuropsychopharmacol. 2020;39:19–28.
Nishitani S, Suzuki S, Ochiai K, Yao A, Fujioka T, Fujisawa TX, et al. Altered epigenetic clock in children exposed to maltreatment. Psychiatry Clin Neurosci. 2021;75:110–2.
Sumner JA, Gambazza S, Gao X, Baccarelli AA, Uddin M, McLaughlin KA. Epigenetics of early-life adversity in youth: cross-sectional and longitudinal associations. Clin Epigenetics. 2022;14:48.
Grasso DJ, Drury S, Briggs-Gowan M, Johnson A, Ford J, Lapidus G, et al. Adverse childhood experiences, posttraumatic stress, and FKBP5 methylation patterns in postpartum women and their newborn infants. Psychoneuroendocrinology. 2020;114:104604.
Serpeloni F, Radtke K, de Assis SG, Henning F, Nätt D, Elbert T. Grandmaternal stress during pregnancy and DNA methylation of the third generation: an epigenome-wide association study. Transl Psychiatry. 2017;7:e1202.
Weder N, Zhang H, Jensen K, Yang BZ, Simen A, Jackowski A, et al. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J Am Acad Child Adolesc Psychiatry. 2014;53:417–424. e5
Saito T, Shinozaki G, Koga M, Tanichi M, Takeshita S, Nakagawa R, et al. Effect of interaction between a specific subtype of child abuse and the FKBP5 rs1360780 SNP on DNA methylation among patients with bipolar disorder. J Affect Disord. 2020;272:417–22.
Yang B-Z, Zhang H, Ge W, Weder N, Douglas-Palumberi H, Perepletchikova F, et al. Child abuse and epigenetic mechanisms of disease risk. Am J Prev Med. 2013;44:101–7.
Radtke KM, Schauer M, Gunter HM, Ruf-Leuschner M, Sill J, Meyer A, et al. Epigenetic modifications of the glucocorticoid receptor gene are associated with the vulnerability to psychopathology in childhood maltreatment. Transl Psychiatry. 2015;5:e571.
Kaufman J, Wymbs NF, Montalvo-Ortiz JL, Orr C, Albaugh MD, Althoff R, et al. Methylation in OTX2 and related genes, maltreatment, and depression in children. Neuropsychopharmacology. 2018;43:2204–11.
Efstathopoulos P, Andersson F, Melas PA, Yang LL, Villaescusa JC, Rȕegg J, et al. NR3C1 hypermethylation in depressed and bullied adolescents. Transl Psychiatry. 2018;8:121.
Sumner JA, Colich NL, Uddin M, Armstrong D, McLaughlin KA. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol Psychiatry. 2019;85:268–78.
Serpeloni F, Radtke KM, Hecker T, Sill J, Vukojevic V, Assis SGde, et al. Does prenatal stress shape postnatal resilience? - An epigenome-wide study on violence and mental health in humans. Front Genet. 2019;10:269.
Li M, Fu X, Xie W, Guo W, Li B, Cui R, et al. Effect of early life stress on the epigenetic profiles in depression. Front Cell Dev Biol. 2020;8:867.
Bartel DP. Metazoan MicroRNAs. Cell. 2018;173:20–51.
Serafini G, Pompili M, Innamorati M, Giordano G, Montebovi F, Sher L, et al. The role of microRNAs in synaptic plasticity, major affective disorders and suicidal behavior. Neurosci Res. 2012;73:179–90.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–24.
Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010;1803:1231–43.
Yoon S, Kim SE, Ko Y, Jeong GH, Lee KH, Lee J, et al. Differential expression of MicroRNAs in Alzheimer’s disease: a systematic review and meta-analysis. Mol Psychiatry. 2022;27:2405–13.
Sabaie H, Gharesouran J, Asadi MR, Farhang S, Ahangar NK, Brand S, et al. Downregulation of miR-185 is a common pathogenic event in 22q11.2 deletion syndrome-related and idiopathic schizophrenia. Metab Brain Dis. 2022;37:1175–84.
Kyzar EJ, Bohnsack JP, Pandey SC. Current and future perspectives of noncoding RNAs in brain function and neuropsychiatric disease. Biol Psychiatry. 2021;91:183–93.
Pisanu C, Severino G, Toma ID, Dierssen M, Fusar-Poli P, Gennarelli M, et al. Transcriptional biomarkers of response to pharmacological treatments in severe mental disorders: a systematic review. Eur Neuropsychopharmacol. 2022;55:112–57.
Geaghan M, Cairns MJ. MicroRNA and posttranscriptional dysregulation in psychiatry. Biol Psychiatry. 2015;78:231–9.
Morgunova A, Flores C. MicroRNA regulation of prefrontal cortex development and psychiatric risk in adolescence. Semin Cell Dev Biol. 2021;118:83–91.
Ragusa M, Santagati M, Mirabella F, Lauretta G, Cirnigliaro M, Brex D, et al. Potential associations among alteration of salivary miRNAs, saliva microbiome structure, and cognitive impairments in autistic children. Int J Mol Sci. 2020;21:6203.
Gupta S, Guleria RS, Szabo YZ. MicroRNAs as biomarker and novel therapeutic target for posttraumatic stress disorder in veterans. Psychiatry Res. 2021;305:114252.
Ye L, Xiao X, Xu Y, Zheng C, Chen S, Luo T, et al. Prelimbic cortex miR-34a contributes to (2R,6R)-hydroxynorketamine-mediated antidepressant-relevant actions. Neuropharmacology. 2022;208:108984.
Sun J, Zhang X, Cong Q, Chen D, Yi Z, Huang H, et al. miR143-3p-mediated neuregulin-1-dependent mitochondrial dysfunction contributes to olanzapine resistance in refractory schizophrenia. Biol Psychiatry. 2022;92:419–33.
Zeng D, He S, Zhao N, Hu M, Gao J, Yu Y, et al. Promoter hypomethylation of miR-124 gene is associated with major depressive disorder. Front Mol Neurosci. 2021;14:771103.
Zhou S, Chen R, She Y, Liu X, Zhao H, Li C, et al. A new perspective on depression and neuroinflammation: Non-coding RNA. J Psychiatr Res. 2022;148:293–306.
Maffioletti E, Bocchio-Chiavetto L, Perusi G, Silva RC, Sacco C, Bazzanella R, et al. Inflammation-related microRNAs are involved in stressful life events exposure and in trauma-focused psychotherapy in treatment-resistant depressed patients. Eur J Psychotraumatol. 2021;12:1987655.
Ortega MA, Alvarez-Mon MA, García-Montero C, Fraile-Martinez O, Lahera G, Monserrat J, et al. MicroRNAs as critical biomarkers of major depressive disorder: a comprehensive perspective. Biomedicines. 2021;9:1659.
Ran L, Kong Y, Xiang J, Zeng Q, Zhang C, Shi L, et al. Serum extracellular vesicle microRNA dysregulation and childhood trauma in adolescents with major depressive disorder. Bosnian J Basic Med. 2022. https://doi.org/10.17305/bjbms.2022.7110.
Lopez JP, Fiori LM, Cruceanu C, Lin R, Labonte B, Cates HM, et al. MicroRNAs 146a/b-5 and 425-3p and 24-3p are markers of antidepressant response and regulate MAPK/Wnt-system genes. Nat Commun. 2017;8:15497.
Dwivedi Y. Evidence demonstrating role of microRNAs in the etiopathology of major depression. J Chem Neuroanat. 2011;42:142–56.
Dwivedi Y. MicroRNAs in depression and suicide: recent insights and future perspectives. J Affect Disord. 2018;240:146–54.
Yoshino Y, Roy B, Dwivedi Y. Altered miRNA landscape of the anterior cingulate cortex is associated with potential loss of key neuronal functions in depressed brain. Eur Neuropsychopharmacol. 2020;40:70–84.
Roy B, Dunbar M, Shelton RC, Dwivedi Y. Identification of microRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacology. 2017;42:864–75.
Rahmani S, Kadkhoda S, Ghafouri-Fard S. Synaptic plasticity and depression: the role of miRNAs dysregulation. Mol Biol Rep. 2022;49:9759–65.
Smalheiser NR, Lugli G, Zhang H, Rizavi H, Cook EH, Dwivedi Y. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS ONE. 2014;9:e86469.
Smalheiser NR, Lugli G, Rizavi HS, Torvik VI, Turecki G, Dwivedi Y. MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects. PLoS ONE. 2012;7:e33201.
Smalheiser NR, Lugli G, Rizavi HS, Zhang H, Torvik VI, Pandey GN, et al. MicroRNA expression in rat brain exposed to repeated inescapable shock: differential alterations in learned helplessness vs. non-learned helplessness. Int J Neuropsychopharmacol. 2011;14:1315–25.
Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA‐mediated down‐regulation of the glucocorticoid receptor. Eur J Neurosci. 2008;27:2250–61.
Kawashima H, Numakawa T, Kumamaru E, Adachi N, Mizuno H, Ninomiya M, et al. Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience. 2010;165:1301–11.
Grieco A, Rzeczkowska P, Alm C, Palmert MR. Investigation of peripubertal expression of Lin28a and Lin28b in C57BL/6 female mice. Mol Cell Endocrinol. 2013;365:241–8.
Sangiao-Alvarellos S, Manfredi-Lozano M, Ruiz-Pino F, Navarro VM, Sánchez-Garrido MA, Leon S, et al. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty. Endocrinology. 2013;154:942–55.
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006.
Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis. 2008;14:27–41.
Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41.
Jin X-F, Wu N, Wang L, Li J. Circulating MicroRNAs: a novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cell Mol Neurobiol. 2013;33:601–13.
Maffioletti E, Tardito D, Gennarelli M, Bocchio-Chiavetto L. Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders. Front Cell Neurosci. 2014;8:75.
Belzeaux R, Bergon A, Jeanjean V, Loriod B, Formisano-Tréziny C, Verrier L, et al. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry. 2012;2:e185.
Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, et al. Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol. 2013;23:602–11.
Fiori LM, Lopez JP, Richard-Devantoy S, Berlim M, Chachamovich E, Jollant F, et al. Investigation of miR-1202, miR-135a, and miR-16 in major depressive disorder and antidepressant response. Int J Neuropsychophrmacol. 2017;20:619–23.
Kuang W-H, Dong Z-Q, Tian L-T, Li J. MicroRNA-451a, microRNA-34a-5p, and microRNA-221-3p as predictors of response to antidepressant treatment. Braz J Med Biol Res. 2018;51:e7212.
McKibben LA, Dwivedi Y. MicroRNA regulates early-life stress-induced depressive behavior via serotonin signaling in a sex-dependent manner in the prefrontal cortex of rats. Biol Psychiatry Glob Open Sci. 2021;1:180–9.
McKibben LA, Dwivedi Y. Early life and adult stress promote sex dependent changes in hypothalamic miRNAs and environmental enrichment prevents stress-induced miRNA and gene expression changes in rats. BMC Genomics. 2021;22:701.
Guo L, Zhu Z, Wang G, Cui S, Shen M, Song Z, et al. microRNA-15b contributes to depression-like behavior in mice by affecting synaptic protein levels and function in the nucleus accumbens. J Biol Chem. 2020;295:6831–48.
Prados J, Stenz L, Courtet P, Prada P, Nicastro R, Adouan W, et al. Borderline personality disorder and childhood maltreatment: a genome‐wide methylation analysis. Genes Brain Behav. 2015;14:177–88.
Cattane N, Mora C, Lopizzo N, Borsini A, Maj C, Pedrini L, et al. Identification of a miRNAs signature associated with exposure to stress early in life and enhanced vulnerability for schizophrenia: New insights for the key role of miR-125b-1-3p in neurodevelopmental processes. Schizophr Res. 2019;205:63–75.
Suderman M, Borghol N, Pappas JJ, Pereira SMP, Pembrey M, Hertzman C, et al. Childhood abuse is associated with methylation of multiple loci in adult DNA. BMC Med Genomics. 2014;7:13.
Dukal H, Frank J, Lang M, Treutlein J, Gilles M, Wolf IA, et al. Newborn females show higher stress- and genotype-independent methylation of SLC6A4 than males. Borderline Personal Disord Emot Dysregul. 2015;2:8.
Wikenius E, Myhre AM, Page CM, Moe V, Smith L, Heiervang ER, et al. Prenatal maternal depressive symptoms and infant DNA methylation: a longitudinal epigenome-wide study. Nord J Psychiatry. 2019;73:257–63.
Cardenas A, Faleschini S, Hidalgo AC, Rifas-Shiman SL, Baccarelli AA, DeMeo DL, et al. Prenatal maternal antidepressants, anxiety, and depression and offspring DNA methylation: epigenome-wide associations at birth and persistence into early childhood. Clin Epigenetics. 2019;11:56.
Coplan JD, Smith ELP, Altemus M, Mathew SJ, Perera T, Kral JG, et al. Maternal-infant response to variable foraging demand in nonhuman primates. Ann N Y Acad Sci. 2006;1071:525–33.
van Oers HJ, de Kloet ER, Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Dev Brain Res. 1998;111:245–52.
Levine A, Worrell TR, Zimnisky R, Schmauss C. Early life stress triggers sustained changes in histone deacetylase expression and histone H4 modifications that alter responsiveness to adolescent antidepressant treatment. Neurobiol Dis. 2012;45:488–98.
Elmer GI, Tapocik JD, Mayo CL, Zanos P, Gould TD. Ketamine metabolite (2R,6R)-hydroxynorketamine reverses behavioral despair produced by adolescent trauma. Pharmacol Biochem Behav. 2020;196:172973.
Wrobel AL, Köhler‐Forsberg O, Sylvia LG, Russell SE, Dean OM, Cotton SM, et al. Childhood trauma and treatment outcomes during mood‐stabilising treatment with lithium or quetiapine among outpatients with bipolar disorder. Acta Psychiat Scand. 2022;145:615–27.
Gröger N, Matas E, Gos T, Lesse A, Poeggel G, Braun K, et al. The transgenerational transmission of childhood adversity: behavioral, cellular, and epigenetic correlates. J Neural Transm. 2016;123:1037–52.
Duempelmann L, Skribbe M, Bühler M. Small RNAs in the transgenerational inheritance of epigenetic information. Trends Genet. 2020;36:203–14.
Goodman SH. Intergenerational transmission of depression. Annu Rev Clin Psychol. 2020;16:213–38.
O’Brien EA, Ensbey KS, Day BW, Baldock PA, Barry G. Direct evidence for transport of RNA from the mouse brain to the germline and offspring. BMC Biol. 2020;18:45.
Monaco AP. An epigenetic, transgenerational model of increased mental health disorders in children, adolescents and young adults. Eur J Hum Genet. 2021;29:387–95.
Lee GS, Conine CC. The transmission of intergenerational epigenetic information by sperm microRNAs. Epigenomes. 2022;6:12.
Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, et al. Intergenerational transmission of maternal childhood maltreatment exposure: Implications for fetal brain development. J Am Acad Child Adolesc Psychiatry. 2017;56:373–82.
Lewis CR, Olive MF. Early-life stress interactions with the epigenome: potential mechanisms driving vulnerability toward psychiatric illness. Behav Pharmacol. 2014;25:341–51.
Funding
This work was supported by grants from the National Institute of Mental Health (R01MH118884; R01MH100616; R01MH124248; R01MH107183; R01MH118884; R01MH130539) to YD.
Author information
Authors and Affiliations
Contributions
Conceptualization, YD; co-written, SO, YD; funding acquisition, YD; review and editing, YD. Both authors have read and agreed to publish the current version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ochi, S., Dwivedi, Y. Dissecting early life stress-induced adolescent depression through epigenomic approach. Mol Psychiatry 28, 141–153 (2023). https://doi.org/10.1038/s41380-022-01907-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01907-x
This article is cited by
-
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
Nature Reviews Gastroenterology & Hepatology (2024)
-
Early Life Stress and Major Depressive Disorder—An Update on Molecular Mechanisms and Synaptic Impairments
Molecular Neurobiology (2024)
-
Vulnerability and resilience to prenatal stress exposure: behavioral and molecular characterization in adolescent rats
Translational Psychiatry (2023)
-
Strong associations of telomere length and mitochondrial copy number with suicidality and abuse history in adolescent depressed individuals
Molecular Psychiatry (2023)