Abstract
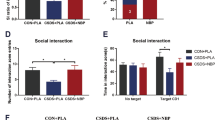
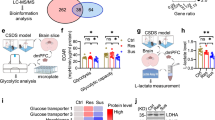
Depression is a mental illness frequently accompanied by disordered energy metabolism. A dysregulated hypothalamus pituitary adrenal axis response with aberrant glucocorticoids (GCs) release is often observed in patients with depression. However, the associated etiology between GCs and brain energy metabolism remains poorly understood. Here, using metabolomic analysis, we showed that the tricarboxylic acid (TCA) cycle was inhibited in chronic social defeat stress (CSDS)-exposed mice and patients with first-episode depression. Decreased mitochondrial oxidative phosphorylation was concomitant with the impairment of the TCA cycle. In parallel, the activity of pyruvate dehydrogenase (PDH), the gatekeeper of mitochondrial TCA flux, was suppressed, which is associated with the CSDS-induced neuronal pyruvate dehydrogenase kinase 2 (PDK2) expression and consequently enhanced PDH phosphorylation. Considering the well-acknowledged role of GCs in energy metabolism, we further demonstrated that glucocorticoid receptors (GR) stimulated PDK2 expression by directly binding to its promoter region. Meanwhile, silencing PDK2 abrogated glucocorticoid-induced PDH inhibition, restored the neuronal oxidative phosphorylation, and improved the flux of isotope-labeled carbon (U-13C] glucose) into the TCA cycle. Additionally, in vivo, pharmacological inhibition and neuron-specific silencing of GR or PDK2 restored CSDS-induced PDH phosphorylation and exerted antidepressant activities against chronic stress exposure. Taken together, our findings reveal a novel mechanism of depression manifestation, whereby elevated GCs levels regulate PDK2 transcription via GR, thereby impairing brain energy metabolism and contributing to the onset of this condition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gururajan A, Reif A, Cryan JF, Slattery DA. The future of rodent models in depression research. Nat Rev Neurosci. 2019;20:686–701.
Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41.
Chen X, Luo J, Leng Y, Yang Y, Zweifel LS, Palmiter RD, et al. Ablation of type III adenylyl cyclase in mice causes reduced neuronal activity, altered sleep pattern, and depression-like phenotypes. Biol Psychiatry. 2016;80:836–48.
Liu F, Shi J, Tanimukai H, Gu J, Gu J, Grundke-Iqbal I, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132:1820–32.
Attwell D, Laughlin S. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45.
Kolar D, Kleteckova L, Brozka H, Vales K. Brain energy metabolism and its role in animal models of depression, bipolar disorder, schizophrenia and autism. Neurosci Lett. 2021;760:136003.
Martin SA, Souder DC, Miller KN, Clark JP, Sagar AK, Eliceiri KW, et al. GSK3β Regulates Brain Energy Metabolism. Cell Rep. 2018;23:1922–31.e1924.
A O,U M, Lf B, A GC. Energy metabolism in childhood neurodevelopmental disorders. EBioMedicine. 2021;69:103474.
Goyal MS, Vlassenko AG, Blazey TM, Su Y, Couture LE, Durbin TJ, et al. Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 2017;26:353–60.e353.
Fang W, Xiao N, Zeng G, Bi D, Dai X, Mi X, et al. APOE4 genotype exacerbates the depression-like behavior of mice during aging through ATP decline. Translational Psychiatry. 2021;11:507.
Wu J, Zhao Y, Park YK, Lee JY, Gao L, Zhao J, et al. Loss of PDK4 switches the hepatic NF-κB/TNF pathway from pro-survival to pro-apoptosis. Hepatology. 2018;68:1111–24.
Ryall JG, Cliff T, Dalton S, Sartorelli V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell. 2015;17:651–62.
Lindqvist D, Wolkowitz O, Picard M, Ohlsson L, Bersani F, Fernström J, et al. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacol. 2018;43:1557–64.
Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychia. 2010;67:360–8.
Okamoto N, Viswanatha R, Bittar R, Li Z, Haga-Yamanaka S, Perrimon N, et al. A membrane transporter is required for steroid hormone uptake in drosophila. Dev Cell. 2018;47:294–305.e297.
Vyas S, Maatouk L. Contribution of glucocorticoids and glucocorticoid receptors to the regulation of neurodegenerative processes. CNS Neurol Disord Drug Targets. 2013;12:1175–93.
Pariante CM. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur Neuropsychopharm. 2017;27:554–9.
Solomon MB, Wulsin AC, Rice T, Wick D, Myers B, McKlveen J, et al. The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Horm Behav. 2014;65:363–71.
Wei Q, Fentress HM, Hoversten MT, Zhang L, Hebda-Bauer EK, Watson SJ, et al. Early-life forebrain glucocorticoid receptor overexpression increases anxiety behavior and cocaine sensitization. Biol Psychiatry. 2012;71:224–31.
Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22:527–36.
Paugh SW, Bonten EJ, Savic D, Ramsey LB, Thierfelder WE, Gurung P, et al. NALP3 inflammasome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells. Nat Genet. 2015;47:607–14.
Roqueta-Rivera M, Esquejo RM, Phelan PE, Sandor K, Daniel B, Foufelle F, et al. SETDB2 links glucocorticoid to lipid metabolism through insig2a regulation. Cell Metab. 2016;24:474–84.
Magomedova L, Cummins CL. Glucocorticoids and metabolic control. Handb Exp Pharmacol. 2016;233:73–93.
Yau JL, Seckl JR. Local amplification of glucocorticoids in the aging brain and impaired spatial memory. Front Aging Neurosci. 2012;4:24.
Park S, Jeon JH, Min BK, Ha CM, Thoudam T, Park BY, et al. Role of the pyruvate dehydrogenase complex in metabolic remodeling: differential pyruvate dehydrogenase complex functions in metabolism. Diabetes Metab J. 2018;42:270–81.
Wang G, Liu X, Xie J, Meng J, Ni X. PDK-1 mediated Hippo-YAP-IRS2 signaling pathway and involved in the apoptosis of non-small cell lung cancer cells. Biosci Rep. 2019;39:BSR20182099.
Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329:191–6.
Jeong JY, Jeoung NH, Park KG, Lee IK. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab J. 2012;36:328–35.
Faron-Górecka A, Kuśmider M, Kolasa M, Żurawek D, Szafran-Pilch K, Gruca P, et al. Chronic mild stress alters the somatostatin receptors in the rat brain. Psychopharmacology. 2016;233:255–66.
Jiang C, Lin WJ, Sadahiro M, Labonté B, Menard C, Pfau ML, et al. VGF function in depression and antidepressant efficacy. Mol Psychiatry. 2018;23:1632–42.
Montagud-Romero S, Blanco-Gandía MC, Reguilón MD, Ferrer-Pérez C, Ballestín R, Miñarro J, et al. Social defeat stress: Mechanisms underlying the increase in rewarding effects of drugs of abuse. Eur J Neurosci. 2018;48:2948–70.
Zou WJ, Song YL, Wu MY, Chen XT, You QL, Yang Q, et al. A discrete serotonergic circuit regulates vulnerability to social stress. Nat. Commun. 2020;11:4218.
Carlson D, David LK, Gallagher NM, Vu MT, Shirley M, Hultman R, et al. Dynamically timed stimulation of corticolimbic circuitry activates a stress-compensatory pathway. Biol Psychiatry. 2017;82:904–13.
Poolman TM, Farrow SN, Matthews L, Loudon AS, Ray DW. Pin1 promotes GR transactivation by enhancing recruitment to target genes. Nucl Acids Res. 2013;41:8515–25.
Vaz-Silva J, Gomes P, Jin Q, Zhu M, Zhuravleva V, Quintremil S, et al. Endolysosomal degradation of Tau and its role in glucocorticoid-driven hippocampal malfunction. EMBO J. 2018;37:e99084.
Hoefs SJ, Dieteren CE, Distelmaier F, Janssen RJ, Epplen A, Swarts HG, et al. NDUFA2 complex I mutation leads to Leigh disease. Am J Hum Genet. 2008;82:1306–15.
Liao EC, Hsu YT, Chuah QY, Lee YJ, Hu JY, et al. Radiation induces senescence and a bystander effect through metabolic alterations. Cell Death Dis. 2014;5:e1255.
Han YM, Kim MS, Jo J, Shin D, Kwon SH, Seo JB, et al. Decoding the temporal nature of brain GR activity in the NFκB signal transition leading to depressive-like behavior. Mol Psychiatry. 2021;26:5087–96.
Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry. 2015;20:755–63.
Schmidt MV, Sterlemann V, Wagner K, Niederleitner B, Ganea K, Liebl C, et al. Postnatal glucocorticoid excess due to pituitary glucocorticoid receptor deficiency: differential short- and long-term consequences. Endocrinology. 2009;150:2709–16.
Díaz-García CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab. 2017;26:361–74.e364.
Zheng H, Yu WM, Shen J, Kang S, Hambardzumyan D, Li JY, et al. Mitochondrial oxidation of the carbohydrate fuel is required for neural precursor/stem cell function and postnatal cerebellar development. Sci Adv. 2018;4:eaat2681.
Zacharias NM, Chan HR, Sailasuta N, Ross BD, Bhattacharya P. Real-time molecular imaging of tricarboxylic acid cycle metabolism in vivo by hyperpolarized 1-(13)C diethyl succinate. J Am Chem Soc. 2012;134:934–43.
Shao WH, Chen JJ, Fan SH, Lei Y, Xu HB, Zhou J, et al. Combined metabolomics and proteomics analysis of major depression in an animal model: perturbed energy metabolism in the chronic mild stressed rat cerebellum. Omics. 2015;19:383–92.
Chen JJ, Xie J, Li WW, Bai SJ, Wang W, Zheng P, et al. Age-specific urinary metabolite signatures and functions in patients with major depressive disorder. Aging. 2019;11:6626–37.
Głombik K, Detka J, Kurek A, Budziszewska B. Impaired brain energy metabolism: involvement in depression and hypothyroidism. Front Neurosci. 2020;14:586939.
Bu Q, Zhang LK, Guo X, Feng Y, Yan H, Cheng W, et al. The antidepressant effects and serum metabonomics of bifid triple viable capsule in a rat model of chronic unpredictable mild stress. Front Nutr. 2022;9:947697.
Shen D, Zhao H, Gao S, Li Y, Cheng Q, Bi C, et al. Clinical serum metabolomics study on fluoxetine hydrochloride for depression. Neurosci Lett. 2021;746:135585.
Kaddurah-Daouk R, Bogdanov MB, Wikoff WR, Zhu H, Boyle SH, Churchill E, et al. Pharmacometabolomic mapping of early biochemical changes induced by sertraline and placebo. Transl Psychiat. 2013;3:e223.
Sullivan CR, O’Donovan SM, McCullumsmith RE, Ramsey A. Defects in bioenergetic coupling in schizophrenia. Biol Psychiatry. 2018;83:739–50.
Ni M, Solmonson A, Pan C, Yang C, Li D, Notzon A, et al. Functional assessment of lipoyltransferase-1 deficiency in cells, mice, and humans. Cell Rep. 2019;27:1376–86.e1376.
Jha MK, Jeon S, Suk K. Pyruvate dehydrogenase kinases in the nervous system: their principal functions in neuronal-glial metabolic interaction and neuro-metabolic disorders. Curr Neuropharmacol. 2012;10:393–403.
Nakai N, Obayashi M, Nagasaki M, Sato Y, Fujitsuka N, Yoshimura A, et al. The abundance of mRNAs for pyruvate dehydrogenase kinase isoenzymes in brain regions of young and aged rats. Life Sci. 2000;68:497–503.
Lee SH, Choi BY, Kho AR, Hong DK, Kang BS, Park MK, et al. Combined Treatment of dichloroacetic acid and pyruvate increased neuronal survival after seizure. Nutrients. 2022;14:4804.
Rahman MH, Bhusal A, Kim JH, Jha MK, Song GJ, Go Y, et al. Astrocytic pyruvate dehydrogenase kinase-2 is involved in hypothalamic inflammation in mouse models of diabetes. Nat Commun. 2020;11:5906.
Halim ND, Mcfate T, Mohyeldin A, Okagaki P, Korotchkina LG, Patel MS, et al. Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia. 2010;58:1168–76.
Castelli V, Benedetti E, Antonosante A, Catanesi M, Pitari G, Ippoliti R, et al. Neuronal cells rearrangement during aging and neurodegenerative disease: metabolism, oxidative stress and organelles dynamic. Front Mol Neurosci. 2019;12:132.
Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19:609–33.
Bolaños JP. Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem. 2016;139:115–25.
Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–42.
Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol. 2010;61:81.
Kim CS, Brager DH, Johnston D. Perisomatic changes in h-channels regulate depressive behaviors following chronic unpredictable stress. Mol Psychiatry. 2018;23:892–903.
Iyo AH, Feyissa AM, Chandran A, Austin MC, Regunathan S, Karolewicz B. Chronic corticosterone administration down-regulates metabotropic glutamate receptor 5 protein expression in the rat hippocampus. Neuroscience. 2010;169:1567–74.
van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, et al. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–8.
Johnson TA, Chereji RV, Stavreva DA, Morris SA, Hager GL, Clark DJ. Conventional and pioneer modes of glucocorticoid receptor interaction with enhancer chromatin in vivo. Nucl Acids Res. 2018;46:203–14.
Magomedova L, Tiefenbach J, Zilberman E, Le Billan F, Voisin V, Saikali M, et al. ARGLU1 is a transcriptional coactivator and splicing regulator important for stress hormone signaling and development. Nucl Acids Res. 2019;47:2856–70.
Kwon HS, Huang B, Unterman TG, Harris RA. Protein kinase B-alpha inhibits human pyruvate dehydrogenase kinase-4 gene induction by dexamethasone through inactivation of FOXO transcription factors. Diabetes. 2004;53:899–910.
Connaughton S, Chowdhury F, Attia RR, Song S, Zhang Y, Elam MB, et al. Regulation of pyruvate dehydrogenase kinase isoform 4 (PDK4) gene expression by glucocorticoids and insulin. Mol Cell Endocrinol. 2010;315:159–67.
Xu Z, Guo X, Yang Y, Tucker D, Lu Y, Xin N, et al. Low-level laser irradiation improves depression-like behaviors in mice. Mol Neurobiol. 2017;54:4551–9.
Moffat C, Pacheco JG, Sharp S, Samson AJ, Bollan KA, Huang J, et al. Chronic exposure to neonicotinoids increases neuronal vulnerability to mitochondrial dysfunction in the bumblebee (Bombus terrestris). Faseb J. 2015;29:2112–9.
Zhu X, Huang Y, Li S, Ge N, Li T, Wang Y, et al. Glucocorticoids reverse diluted hyponatremia through inhibiting arginine vasopressin pathway in heart failure rats. J Am Heart Assoc. 2020;9:e014950.
Perry R, Wang Y, Cline GW, Rabin-Court A, Song J, Dufour S, et al. Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell. 2018;172:234–48.e217.
Abemayor E, Kovachich GB, Haugaard N. Effects of dichloroacetate on brain pyruvate dehydrogenase. J Neurochem. 1984;42:38–42.
Check JH, Wilson C, Cohen R, Sarumi M. Evidence that mifepristone, a progesterone receptor antagonist, can cross the blood brain barrier and provide palliative benefits for glioblastoma multiforme grade IV. Anticancer Res. 2014;34:2385–8.
Acknowledgements
We thank all volunteers, patients, and their families for their contribution in this study.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81602846; No. 82272253), Natural Science Foundation of Shandong Province (No. ZR2021MH145), Taishan Scholar Project of Shandong Province (No. tsqn201812159), China International Medical Foundation (No. Z-2018-35-2002), Research Fund for Lin He’s Academician Workstation of New Medicine and Clinical Translation of Jining Medical University (No. JYHL2021FMS19), and Key Research and Development Projects of Jining City (No. 2021YXNS084).
Author information
Authors and Affiliations
Contributions
CW and CC wrote the manuscript. PX and LZ performed the in vitro experiments. CW, CC and HX conducted the in vivo experiments. BC and PJ conducted data analysis. PJ contributed to study conception and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Cui, C., Xu, P. et al. Targeting PDK2 rescues stress-induced impaired brain energy metabolism. Mol Psychiatry 28, 4138–4150 (2023). https://doi.org/10.1038/s41380-023-02098-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02098-9