Abstract
Atherosclerosis is a chronic inflammatory vascular disease driven by traditional and nontraditional risk factors. Genome-wide association combined with clonal lineage tracing and clinical trials have demonstrated that innate and adaptive immune responses can promote or quell atherosclerosis. Several signaling pathways, that are associated with the inflammatory response, have been implicated within atherosclerosis such as NLRP3 inflammasome, toll-like receptors, proprotein convertase subtilisin/kexin type 9, Notch and Wnt signaling pathways, which are of importance for atherosclerosis development and regression. Targeting inflammatory pathways, especially the NLRP3 inflammasome pathway and its regulated inflammatory cytokine interleukin-1β, could represent an attractive new route for the treatment of atherosclerotic diseases. Herein, we summarize the knowledge on cellular participants and key inflammatory signaling pathways in atherosclerosis, and discuss the preclinical studies targeting these key pathways for atherosclerosis, the clinical trials that are going to target some of these processes, and the effects of quelling inflammation and atherosclerosis in the clinic.
Similar content being viewed by others
Introduction
Atherosclerosis is the process of plaque formation including various cells, lipids, and debris tissue in the vascular intima,1 which is identified as a chronic vascular inflammation mediated by traditional and nontraditional risk factors.2 Atherosclerosis was traditionally regarded as a disease of cholesterol accumulation caused by the retention of lipoproteins including low-density lipoprotein (LDL) in the intimal of arteries. LDL taken up by scavenger receptor induces the continuous immune cell infiltration into the atherosclerotic plaque.3,4,5,6 The hypothesis that atherosclerosis is an inflammatory disease was firstly suggested by Russell Ross in 1999,7 based on observations that circulating monocytes infiltrate into the developing fatty streak. The antigens involved in inflammation initiation in atherosclerosis are only recently beginning to be elucidated. Genome-wide association combined with clonal lineage tracing and clinical trials have identified that the mechanisms of innate and adaptive immunes can promote or quell atherosclerosis.8 Much evidence suggests that potential major antigens involved in atherosclerosis include neoepitopes generated by oxidized LDL (oxLDL) formed in the vessel wall or when cells undergo apoptotic death.9 In addition, other potential antigens released from apoptotic cells in the plaques can further promote the progression of the atherosclerotic plaque, and impaired apoptotic cell clearance can sustain atherogenesis.10 Dysregulation of immune cells in the plaques has been recently uncovered by using single-cell transcriptomic and proteomic analyses.11 The plaques in symptomatic patients exhibited the characterization of a distinct CD4+ T-cell subset and T cells to be activated and differentiated, whereas in the plaques from asymptomatic patients, T cells and macrophages were also activated and raised interleukin-1β (IL-1β) signaling. Altogether these observations underscore the diversity of phenotype and functions of immune cells in atherosclerotic plaques and the interplay between systemic immune response and local event at the plaque site acts as drivers of plaque instability.
New evidence suggests that remnants of triglyceride-rich lipoproteins promote the development of atherogenesis, highlighted by deleterious effects of apolipoprotein (Apo) CIII.12 Based on the intimate relationship between lipids and inflammation, a metabolic-immune hypothesis of atherosclerosis has recently been proposed aiming to provide a complementary view regarding the effect of lipids and inflammation on the pathogenesis of atherosclerosis.13 Therefore, inflammation can drive vascular hyperplasia without traditional cardiovascular risk factors and involves aspects of plaque biology that lead to the complications of advanced atherosclerosis.
Given the relationship between inflammation and atherosclerosis, treatment of atherosclerosis from an inflammatory perspective appears to be a more effective anti-atherosclerotic modality. Although no direct evidence supports that selectively intervention of inflammation can improve outcomes in atherosclerosis patients,14 clinical trials have unequivocally shown that modulation of inflammation can forestall atherosclerosis and its complications,15,16 which represents the transformation of inflammation in atherosclerosis from theory to practice.14 Therefore, a gamut of attractive therapeutic strategies for modulation of inflammation has been suggested in the treatment of atherosclerosis, including inhibiting pro-inflammatory cytokines, blocking key inflammatory signaling pathways, and promoting inflammatory resolution.2 In addition, new immunotherapies for atherosclerotic cardiovascular events could be identified by clarifying the dysregulation of specific immune within the plaque regions, beyond the traditional management of cardiovascular risk factors and the use of standard lipid-lowering agents.11
In this review, we summarize the knowledge on cellular participants and inflammatory signaling pathways in atherosclerosis, and discuss these pathways as potential therapeutic targets for atherosclerosis and clinical trials that are doing targeting some of these processes, and the effects of quelling inflammation and atherosclerosis in the clinic. Much of our understanding of the roles of pivotal inflammatory signaling pathways in atherosclerosis is based on findings from the most recent experimental studies and clinical trials. Although both suppressing inflammation and promoting resolution may lead to unwanted effects, taming inflammation might substantially offer multiple benefits for human health.2
Cells involved in atherosclerosis
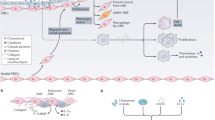
Atherosclerotic plaques are complex structures that consist of vascular cells and immune cells. In this section, we discuss how various types of cells contribute to vascular inflammation and key cell types involved in atherosclerosis and key roles (Fig. 1).
Key cells involved in atherosclerosis. a Phenotypic switching of VSMCs in atherosclerosis. In the healthy arterial wall, VSMCs are a contractile phenotype expressing contractile proteins (ACTA2, SM22α, Myocardin (MYOCD), and MYH11). Upon PDGF-BB and TNF-α, VSMCs switch to a synthetic phenotype, which increases the production of ECM, exosomes, pro-inflammatory cytokines, and MMPs. VSMCs release calcifying vesicles to propagate vascular calcification. KLF4 promotes phenotypic switching of VSMCs into foam-like, macrophage-like, osteochondrocyte-like, adipocyte-like, and Sca1+ mesenchymal-like VSMCs. Shear stress induces transdifferentiation of VSMCs into endothelial-like cells. The transcription factors TCF21 and OCT4 (octamer binding transcription factor) promote modulation of VSMCs into atheroprotective myofibroblast-like phenotype. b Plasticity and function of macrophages in atherosclerosis. Monocytes differentiate toward various phenotypes of macrophages in response to stimuli in atherosclerotic lesions. Among them, M1 macrophages secret pro-inflammatory cytokines; M2, M (Hb), and Mhem phenotypes are anti-inflammatory; Mox macrophages exhibit an antioxidant effect; and M4 phenotypes express pro-inflammatory cytokines and have impaired phagocytosis. c Lymphocytes in atherosclerosis. CD4+ T cells can differentiate into distinct lineages including T helper 1 (Th1), Th2, Th17, T regulatory (Treg), and many other Th cells. Th1 cells produce TNF-α and IFN-γ, indicating a pro-inflammatory and pro-atherogenic role of Th1 cells. Treg cells promote inflammatory resolution and dampen atherosclerosis progression via the production of IL-10 and TGFβ. The effect of Th2, Th9, and Th17 cells on the development of atherosclerosis remains controversial. B cells can exert both a pathogenic and protective role in atherosclerosis. B cells have two main subsets B1 and B2 cells. B1 cells exert an atheroprotective effect by the release of IgM antibodies against oxidation-specific epitopes. Similarly, Breg cells also act atheroprotective by the production of IL-10. B2 cells exhibit both pro-atherogenic and atheroprotective depending on the inflammatory microenvironment
Vascular cells
Vascular endothelial cells
Endothelial barrier integrity plays a key role in maintaining a fluid balance between the circulation and tissues and vascular homeostasis. Studies indicate an association between endothelial dysfunction, subsequent elevation in the levels of endothelial factors, and development and intensification of coronary artery disease and atherosclerosis.17 As secretory cells, endothelial cells (ECs) exert significant paracrine and endocrine actions through their influence on the underlying VSMCs or on circulating blood elements, such as platelets and white blood cells. They produce and release a variety of vasoactive substances, such as endothelin-1 (ET-1), nitric oxide (NO), prostacyclin, angiotensin 2 (Ang II), vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), as well as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), which regulate vasodilation, platelet aggregation, and monocyte infiltration.18 The mitochondrial ubiquitin ligase MARCH5, a critical regulator of mitochondrial dynamics, mitophagy, and apoptosis, is demonstrated to protect endothelial function against hypoxia/ischemic injury via serine/threonine kinase AKT/eNOS (endothelial NO synthase) axis.19 Inhibition of receptor-type vascular endothelial protein tyrosine phosphatase (VE-PTP) elicits phosphorylation of eNOS to break endothelial dysfunction.20 β-catenin facilitates endothelial survival by promoting activation of eNOS and expression of the flow-dependent anti-apoptotic gene.21 The beneficial effect of NO is partially mediated by inhibiting the expression of MCP-1 (monocyte chemoattractant protein-1).22 Thus, regulation of endothelial integrity and function is necessary for maintaining vascular homeostasis and for preventing the development of atherosclerosis.
Vascular smooth muscle cells
VSMCs are a major type of cells presented at all stages of atherosclerotic development. Substantial heterogeneity of VSMCs in morphology and gene expression related to atherosclerosis has been identified by transcriptional profiling of healthy arteries, including observation of atypical, rare VSMCs with Sca1 positive and VSMCs with expressing genes related to phenotypic switching, suggesting that there are VSMC subtypes relevant to a particular disease.23,24 Phenotypically modified VSMCs can produce a large number of extracellular matrix (ECM) proteins (such as elastic fibers, collagens, proteoglycans, and MMPs), and secrete a wide range of cytokines (MCP-1, IL-1β, and IL-6) that can regulate the function of neighboring cells in a paracrine manner, and release various extracellular vesicles (EVs) to induce vascular calcification.25,26
VSMCs play both beneficial and detrimental roles in the development of atherogenic plaques, which depends on the nature of their phenotypic changes. Besides synthetic phenotype, VSMCs adopt another phenotype, such as pro-inflammatory macrophage-like phenotype,23,27 mesenchymal stem cell-like phenotype,23 fibromyocyte phenotype,24 osteogenic phenotype,28 EC-like,29 adipocyte-like,30 and intermediate cell phenotype31 during the development of atherosclerotic disease (Fig. 1a). Although VSMCs can return to the contractile state under some conditions,31,32 to date, no evidence shows that VSMCs in the intima can relocalize in the media. The wide differences of VSMC phenotypes between medial, intimal, and plaque could influence the ultimate role of VSMCs in atherosclerosis.33
Although the prevailing point is that VSMCs exert beneficial roles in advanced atherosclerosis as they can stabilize fibrous cap and are major cells producing ECM, single-cell RNA-sequencing combination with lineage tracing have revealed much more-diverse phenotypes of VSMCs in the various stages of atherosclerosis.1,2 Lipid uptake alters the VSMC phenotype into macrophage-like and foam-like.34 The detrimental effects of these macrophage-like VSMCs have been supported by the specific deletion of KLF4 (transcription factor krueppel-like factor 4) in VSMCs.35 The macrophage-like or foam-like VSMCs are pro-inflammatory and contribute to plaque vulnerability (Fig. 2).
Overview of inflammatory responses in atherosclerotic development. At the early stages, activated platelets mediate firm adhesion between platelets, leukocytes, and the vascular endothelium by secreting platelet-activating factor (PAF). In progressing plaques, VSMCs migrate from the medial to the subendothelial space where they undergo proliferation and developing fibrous cap. OxLDL induces phenotype switching of VSMCs to synthetic, macrophage-like VSMCs and the formation of foam cells. Excessive deposition of lipids triggers VSMC apoptosis and senescence, leading to necrosis. At predilection sites with the disturbed flow, neutrophil-released neutrophil extracellular traps (NETs) induce desquamation of endothelial cells and lead to rapid occlusion of the affected vessels. Monocyte-derived macrophages ingest oxLDL and release pro-inflammatory cytokines. Excessive deposition of lipids, as well as cytokines and histamine released from dendritic cells and mast cells, trigger the proliferation, polarization of macrophages, or even cell death
Smooth muscle 22 alpha (SM22α), an important cytoskeleton-associated protein, is required for maintaining the contractile phenotype of VSMCs and is a sensitive and specific marker for identifying the dedifferentiation and phenotypic switching of VSMCs.36,37,38 Recent studies from us and others have indicated that SM22α binds and regulates the organization of actin cytoskeleton,38,39 and maintains Ang II-activated extracellular signal-regulated kinase (ERK) 1/2 contraction signaling by mediating mitogen-activated protein kinase (MAPK) phosphatase 3 ubiquitination degradation, and thus modulating the VSMCs phenotype in the physiological state.40 In addition, SM22α also acts as an adapter or scaffold protein to modulate the formation and translocation of signaling complexes, and to maintain adaptive phenotype alteration of VSMCs. The arteries of Sm22α−/− mice develop enhanced inflammatory response and oxidative stress, which contributes to neointimal hyperplasia through different signaling mechanisms,41,42,43 suggesting that Sm22α−/− VSMCs have transited to a synthetic or pro-inflammatory state.44 A more recent study revealed that SM22α inhibits abdominal aortic aneurysm formation by preventing VSMC phenotypic switching by suppressing ROS/nuclear factor (NF)-κB.45 These findings support the notion that molecular changes by SM22α loss may have already initiated the early inflammation of atherosclerosis.46
Transcriptome profiling reveals an increased tendency to develop atherosclerosis in SM22α-deficient mice.46 SM22α mediates suppression of VSMC proliferation and neointima hyperplasia via blockade of Ras-ERK1/2 pathway47 and inhibits migration via uncoupling Ras-Arp2/3 interaction in synthetic VSMCs.48 Phosphorylation of SM22α facilitates Ang II-induced ROS production via activating protein kinase Cδ (PKCδ)-p47phox axis in actin dynamics-dependent manner, contributing to hypertrophy and hyperplasia of VSMCs in vitro and in vivo.49 We also demonstrated that insulin-independent glucose transporter type 4 (GLUT4) translocation in proliferative VSMCs involves SM22α.50 Furthermore, TNF receptor-associated factor 6 (TRAF6)-mediated SM22α ubiquitination promotes activation of glucose-6-phosphate dehydrogenase (G6PD) and reduces the production of nicotinamide adenine dinucleotide phosphate, leading to impaired glutathione (GSH) homeostasis and VSMC survival.51 In addition, we have indicated that deficiency of SM22α enhances the interaction between VSMCs and macrophages and triggers VSMC apoptosis via promoting zinc finger protein-36 (ZFP36)-mediated decay of Bcl-2 mRNA.44 More recently, we elucidated the roles of SM22α in nuclear receptor LXRα (liver X receptor α)-modulated cholesterol homeostasis of VSMCs.52 Loss of SM22α blocked LXRα nuclear import and reduced ATP-binding cassette transporter (ABCA1)-driven cholesterol efflux via promoting F-actin depolymerization, which aggravated the development of atherosclerosis. Despite protecting against vascular inflammation and oxidative stress, accumulation of SM22α protein accelerates senescence of VSMCs and vascular aging via suppression of murine double minute 2 (Mdm2)-mediated degradation of p53 in vitro and in vivo.53 Collectively, SM22α is involved in various VSMC behaviors via regulating different signaling pathways and plays important roles in the pathogenesis of atherosclerosis.54
Fibroblasts
Fibroblasts in the adventitia are metabolically active cells and play a prominent role in the development of atherosclerosis.55 Latest studies have indicated that the adventitia provides a dynamic microenvironment for the regulation of both structural and functional properties of all three arterial layers. Importantly, resident adventitial fibroblasts may be the first cells in the vascular wall in response to inflammatory and environmental stimuli.56 Activated fibroblasts enhance interaction with ECs and VSMCs and regulate their functions, as well as recruit immune cells into the vessel wall.43 The most important roles of fibroblasts in advanced atherosclerosis involve modulation of the inflammatory response and ECM protein production, and maintenance of the structural integrity of the plaque and balance of MMP production, to promote beneficial tissue remodeling, alongside preventing plaque rupture.55 Regulation of fibroblast activities to control or reverse the progression of atherosclerosis may be an attractive target for therapeutic intervention.
Platelets
Platelets are central to inflammation-related manifestations of atherosclerosis. Beyond effects on the immune cell infiltration, platelets regulate the metabolism of cholesterol by modifying, binding and endocytosing LDL via scavenger receptors and promoting the formation of macrophage foam cells.57 The platelet-activating factor (PAF) released from platelets can induce integrins to mediate firm adhesion between the endothelium and leukocytes or platelets (Fig. 2).58 Platelet-derived extracellular vesicles (PEV) produced by activated platelets can trigger the initiation of atherosclerosis.59 Ablation of platelet apoptosis can reduce atherosclerosis in diabetes mice, leading to a more stable plaque by preventing platelet-monocyte interactions and subsequently monocyte activation.60 The current advances in anti-inflammatory therapies have identified an inflammatory mediator effect of thrombosis secondary to platelet activation.61
Immune cells
Atherosclerosis is multifacetedly triggered by contributions of the immune system in the circulation and at the local vascular lesions. Single-cell proteomics combined with transcriptomic analyses revealed specific characteristics of immune cell dysregulation within the plaques that lead to clinical ischemic stroke or myocardial infarction.11
Monocytes/macrophages
Macrophages in plaques are not exclusively derived from monocytes, but mainly rely on their own local proliferation.62 Macrophages exert atheroprotective effects under homeostatic conditions, through both the clearance of lipoprotein by endocytosis and apoptotic cells by phagocytosis or efferocytosis, thereby abolishing the inflammatory processes involved in plaque formation.63 Macrophages, as a major source of chemokines, cytokines, matrix protein-degrading enzymes, play a critical role in sustained local inflammatory responses and plaque rupture.64 Apoptotic cell-derived nucleotides activate the proliferation of efferocytosis macrophages, which is essential for inflammation resolution and atherosclerosis regression, which may be new ways to treat non-resolving inflammatory diseases.65
Macrophages can be categorized into M1 (classically activated) macrophages that contribute to tissue destruction and secrete pro-inflammatory factors and M2 (alternatively activated) macrophages that produce anti-inflammatory factors. Distinct macrophage subsets resolved at the single-cell transcriptional level revealed activated and pro-inflammatory functional phenotypes, which is not accurately defined by the M1 and M2 phenotypes.11 Furthermore, additional plaque-specific macrophage phenotypes are recently identified, including Mhem, Mox, and M4 (Fig. 1b). Hemorrhage-residing Mhem macrophages participate in hemoglobin clearance via phagocytosis of erythrocyte and exhibit increased cholesterol efflux, which is a subset of atheroprotective and resistant to foam cell formation,66 as they highly express the cholesterol transporters ABCA1 and ABCG1 and the nuclear receptors, LXR-α and LXR-β. Mox macrophages, a pro-atherogenic subset induced by oxidized phospholipids, protect from oxidative stress. M4 macrophages displayed reduced phagocytic capacity, increased neutrophil recruitment, and more effective neutrophil extracellular trap (NET) induction,67 which represents an atherogenic phenotype. In addition, iron accumulation in the plaques may induce the M (Hb) macrophage differentiation, which expresses CD163 (scavenger receptor cysteine-rich type-1 protein M130) and has, therefore, protective properties in atherosclerosis. Furthermore, a novel transcriptional intermediary state between nonpolarized (M0) and inflammatory M1-like macrophages were identified. These macrophages were characterized by the high expression of GATA2, a hematopoietic transcription factor, leading to impaired efferocytosis and efferosome maturation during the earliest stages of atherosclerotic development in humans.68
Collectively, the functions and phenotypes of macrophages can vary widely based on several factors, including the macrophage origin, the stage of atherosclerosis, and the microenvironment69 (Fig. 2). Such determinants not only confer the appearance and functionality of macrophages but also define their heterogeneity at a single-cell level, suggesting that different subsets coexist within one tissue.2 Given the key role of macrophages in atherosclerotic initiation, progression, and resolution, nanoparticle (NP)-based imaging modalities specifically targeting macrophages, as novel diagnostic and therapeutic strategies, are used to therapeutically manipulate macrophages in the plaques to increase plaque stability and to reduce the risk of cardiovascular disease.70,71 For example, a small interfering RNA (siRNA) NP targeting macrophage Ca2+/calmodulin-dependent protein kinase has been shown to improve all signs of plaque stability in advanced atherosclerosis of Ldlr−/− mice72 via an increase in expression of MER tyrosine-protein kinase to drive inflammation resolution.73
Lymphocytes
Increased number of lymphocytes is well identified as an independent risk factor for atherosclerotic disease,74 and reduced lymphocyte number is also considered to be associated with increased risk,75 suggesting the complex role of the immune system in the development of atherosclerosis.76
T cells play a critical role in cellular immunity, including CD4+, CD8+, natural killer (NK) T cells, and helper T cells etc. Most cytokines are secreted by T cells. CD4+ T, termed as T helper (Th) cells, are a heterogeneous group and can differentiate into Th1, Th2, Th17, Treg, and many other Th cells.77 Th1 cells release TNF-α and IFN-γ, exerting a pro-atherogenic role.78 Th2 cells that are a lineage of CD4+ T cells, primarily interact with B cells and produce IL-4, −5, and −13. Based on both pro- and anti-atherogenic actions of Th2 cells, the effect of these cells on atherosclerosis appears to be more complex compared with Th1 cells.79 Th9 cells can produce IL-9 upon transforming growth factor (TGF)-β and IL-4 stimulation. However, it is unknown that Th9 cells are pro-atherogenic or anti-atherogenic.80 Th17 cells release IL-17A, F, and IL-22, which are pro-inflammatory. Despite protecting against fungal and bacterial infections,81 however, the role of Th17 cells in atherosclerotic diseases remains controversial as both pro-atherogenic82,83 and anti-atherogenic actions84 have been reported.
There are very low numbers in regulatory T cells (Tregs) in the plaques, compared with other chronic inflammatory tissues,85 representing an increased local inflammation in the plaques. The anti-inflammatory cytokines TGF-β, IL-10, and IL-35 are important for Treg cell suppression.86 Adoptive transfer of chemokine (C-X3-C motif) receptor-1 (CX3CR1) transduced-Treg cells improve homing to the plaques and dampen progression of atherosclerosis.87 Recently identified Treg cell-related biomarkers in deteriorated atherosclerosis were used to distinguish patients with myocardial infarction from those with stable coronary disease.88 Recently, developments in high-parametric cell immunophenotyping by single-cell RNA-sequencing, mass cytometry, combined tools exploring antigen-specificity reveal that pathogenic ApoB-reactive T cells evolved from immunosuppressive and atheroprotective CD4+ Treg cells and lose their protective properties over time.89
The CD8+ T cell in human and mouse atherosclerosis revealed activated, cytotoxic, dysfunctional, and exhausted cell phenotypes.90 CD8+ Treg cells play a protective effect in advanced atherosclerosis through limiting increases in Th1 cells and macrophages.91 In addition, adoptively transferred CD8+ T cells promote the protective effects of peptide immunization on atherosclerosis in ApoE−/− mice.92
Collectively, Th1 cells play pro-inflammatory and pro-atherogenic, whereas the effects of Th2, Th9, and Th17 cells on the development and progression of atherosclerosis remain controversial.80 Treg cells suppress the activity of CD4+ Th and cytotoxic CD8+ T cells and promote the anti-inflammatory phenotype of macrophages and resolution of inflammation (Fig. 1c).93 CD4+ and CD8+ T cells in the plaques from symptomatic patients with recent cardiovascular events were activated, differentiated, and exhausted compared with their blood counterparts.11 The dynamic balance between different T-cell subsets controls the formation of vulnerable and obstructive plaques during atherosclerotic development.94
B cells can reveal both protective and pathogenic effects in atherosclerosis. B1 and B2 cells are two main subsets identified in human atherosclerotic plaques.95 New observations from the effects of the therapies targeting B cells in autoimmune diseases suggest that the distinct B-cell subsets and different immunoglobulins play a prominent role with atherogenic and protective effects, and it is observed that B-cell subset depleting (modifying) therapies may exert the beneficial side effects on atherosclerotic concomitant disease.96 Besides the production of antibodies, B cells also play regulatory roles through the production of IL-10 and therefore these cells are named regulatory B cells.97,98 Recent studies demonstrate that a low number of IL-10+ B cells in atherosclerosis patients is associated with inflammatory condition99 and that alarmin-activated B cells accelerate atherosclerosis after myocardial infarction via plasma cell-immunoglobulin dependent mechanisms.100 Hence, the identification of human B-cell subsets and their production of IL-10 would help to better understand the role of these cells in atherosclerosis, and to distinguish which of these subsets truly have a pro- or anti-atherogenic role (Fig. 1c).
Neutrophils
Neutrophils are the most abundant circulating white blood cells in humans, have received far less notice until recently. Neutrophils localize at sites of plaque erosion,101 which correlates negatively with endothelial continuity.102 Neutrophil factors attract and activate macrophages. In response to disease stimuli, neutrophils undergo a specialized series of reactions that eventually lead to NETs formation, a complex structure composed of nuclear chromatin and proteins of nuclear cytoplasmatic and granule origin.103 NETs underlie the communication between neutrophils and monocytes directly to stimulate cells into the atherosclerotic plaques and modulate the inflammatory response.104,105 Extracellular cholesterol crystals induce neutrophils to release NETs, which trigger macrophages to express a precursor form of pro-IL-1β. The striking correlation between NETs and dying SMCs, necrotic core sizes, and thin fibrous caps observed in the atherosclerotic mice suggests a direct cytotoxic action of NETs (Fig. 2),106 which accelerate atherosclerosis during endotoxinemia.107
Mast cells
Mast cells are migrant cells in connective tissue with many granules rich in histamine and heparin. Accumulated studies have confirmed that mast cells differentiated from hematopoietic stem cells are also critical for atherosclerosis. They are present in the intimal and epicardial plaques of the aorta in patients, and the number increases with the development of atherosclerosis.108 Mast cells are amplified by self-cascade, increasing leukocyte infiltration and further increasing plaque area as they were degranulated later.109 Protease and Histamine mediators secreted by mast cells mediate apoptosis of ECs, VSMCs, and macrophages (Fig. 2). These cells are rich in trypsin-like enzymes that degrade ApoE and ApoA1, reduce intracellular cholesterol efflux, promote the transforming of macrophages and VSMCs into foam cells, and promote the development of atherosclerosis eventually.110
NK cells
NK cells play an immunoregulatory role in the pathogenesis of atherosclerosis.111 CD160, a unique activating NK cell receptor, can mediate cytolytic responses and production of cytokines.112 Symptomatic plaques of carotid atherosclerosis are associated with increased NK cell infiltration and higher levels of serum NK-activating receptor ligands.113 However, there is still no consensus on whether the role of NK cells in atherosclerosis is related to their cytolytic activity or rather to cytokine secretion. Initial studies showed that depletion of NK cells resulted in decreased atherosclerosis. A recent study demonstrated that hyperresponsiveness or genetic depletion of NK cells do not affect development of atherosclerosis.114 As a whole, the effects of NK cells on the development of atherosclerotic plaques began to be concerned about recently, and however, the conclusions seem to be conflicting and need to be further investigated.115
Dendritic cells
Dendritic cells (DCs) have multiple roles in the development of atherosclerosis in both direct and indirect manners. DCs uptake lipids and form foam cells, contributing to an atherosclerotic plaque at the early stage. Furthermore, the mature DCs can promote activation and proliferation of T cells via presenting antigens to T cells and clear apoptotic cells by efferocytosis. In addition, DCs also regulate the activity of other immune cells by production of cytokines. For example, DCs can recruit circulating haematopoietic cells or leukocytes to the injured vascular sites via secreting chemokines.116 The conditioning cytokines produced by DCs control the differentiation of T cells into various T effectors (Th1, Th2, Th17, and Treg), regulate the activation of B cells and cytotoxic T cells, and polarization of macrophages, which ultimately lead to immune destruction of vascular regions and the onset of atherosclerosis. The study in Ldlr−/− mice found that Atg16l1-deficient CD11b+ DCs produced a TGF-β-dependent tolerance phenotype and promoted CD4+ Treg cell expansion, reducing the development of atherosclerosis.117 Recent studies have reported that Alisol B 23-acetate (23B), a new promoter for cholesterol efflux in DCs, alleviates inflammation and dyslipidemia in advanced atherosclerosis of mice, thereby controlling the atherosclerotic inflammatory state.118 These findings expand our understanding of how DCs affect atherosclerosis and provide new potential approaches to prevent atherosclerosis.
Signaling pathways in atherosclerosis
The signaling pathways mediated by immune, inflammatory mediators are implicated within the atherosclerotic lesion. Understanding these processes helps researchers to create a range of novel biomarkers and treatment modalities. Herein, we mainly give the idea about the important signaling pathways involved in atherosclerosis (Fig. 3), and discuss the recent preclinical studies targeting some of these processes.
Key signaling pathways in atherosclerosis. a TLR signaling pathway. TLR stimulation triggers MyD88 to interact with IRAK4 (interleukin-1 receptor-associated kinase 4), which transmits signals into NF-κB and MAPK to activate the expression of inflammatory cytokines via the MyD88-dependent pathway. Endosomal TLRs transmit signals through the TRIF-dependent pathway. TRIF together with RIP1, TRAF6, and Pellino-1 activate TAK1 or with noncanonical TBK1 (Tank-binding-kinase 1) and IKKε (IKKi) activates interferon regulatory factor 3 (IRF3) and 7 to induce inflammatory cytokines. b NLRP3 inflammasome pathways. The activation of NLRP3 inflammasome has two steps: the priming and the activation. The priming is triggered by endogenous cytokines or microbial molecules. The activation of NLRP3 inflammasome includes canonical and noncanonical pathways. The canonical activation induces caspase-1 activation, which processes pro-IL-1β/pro-IL-18 to IL-1β/IL-18 active form. The noncanonical activation induced by mouse caspase-11, and human caspase-4 and caspase-5, indirectly promotes the expression of pro-IL-1β/pro-IL-18. Activated caspase-1 and caspase-11 can cleave GSDMD (gasdermin D), leading to the formation of pores in the plasma membrane and causing pyroptosis and the release of IL-1β/IL-18. c PCSK9 pathways. The expression of PCSK9 can be activated by oxLDL, LPS, and pro-inflammatory cytokines in ECs, VSMCs, and macrophages. In the absence of PCSK9, the LDL–LDLR complex is internalized. Subsequently, internalized LDLR-LDL-C complex dissociates and LDLR is recycled to the cell surface, whereas LDL-C is directed to lysosomes for degradation. PCSK9 can mediate internalized LDLR-LDL-C complex to degrade, and promote oxLDL-induced inflammation through increasing expression of LOX-1 and TLR4, which increases oxLDL uptake and upregulates inflammatory cytokine expression via activation of ROS and NF-κB
Toll-like receptors
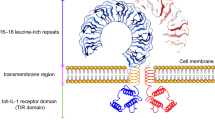
The toll-like receptors (TLRs) are a class of transmembrane proteins that include a cytoplasmic region homologous to the IL-1 receptor and an extracellular leucine-rich domain. Although TLRs are extensively expressed in immune cells such as monocytes, macrophages, DCs, neutrophils, lymphocytes, and vascular cells such as fibroblasts, ECs, VSMCs, the specific TLRs in each cell type play a unique role in the immune response.119
Classification and function of TLRs
TLR signaling induces the production of anti-microbial peptides, pro-inflammatory cytokines, adhesion molecules, reactive nitrogen, and oxygen species. In mammals, a total of 13 TLRs have been identified to date, among which TLR1-10 functions in humans and TLR12-13 in mice,120 and each TLR has the specificity for different ligands to a certain degree.121 For example, heterodimerized TLR2/TLR1 binds to triacylated lipopeptides derived from mycoplasma and Gram-negative bacteria, and diacylated lipopeptides from Gram-positive bacteria are recognized by TLR2/TLR6 heterodimers. TLR3 can recognize siRNA, double-stranded RNA, and self RNA released by injured cells. TLR4 recognizes exogenous and endogenous ligands, including oxLDL, heparan sulfate, HSPs, fibrinogen, hyaluronan fragments, beta-defensin, lipoteichoic acid (LTA), lipopolysaccharide (LPS), protein F, and envelope glycoprotein, that are released at chronic inflammation sites in response to tissue stress or damage, such as atherosclerosis (Fig. 3a).122
TLR signaling pathways
TLR signaling pathways are classified as a MyD88 (myeloid differentiation primary response protein 88)-dependent pathway to trigger NF-κB activation and a TRIF (TIR-domain-containing adaptor protein inducing IFNβ)-dependent pathway. MyD88 is required for all TLRs (except TLR3) and members of IL-1 receptor family, and activates NF-κB and MAPK signaling pathways and inflammatory cytokine expression. TLR activation triggers the interaction of MyD88 with interleukin-1 receptor-associated kinase 4, which is the most upstream serine/threonine kinase of the complex.123 In addition to the classical MyD88-dependent pathway, endosomal TLRs can also transmit signals through the TRIF-dependent pathway. TRIF with TRAF6 (TNF receptor-associated factor 6), TRADD (TNFR1-associated death domain protein), RIP1, and Pellino-1 forms a multiprotein signaling complex that activates NF-κB, MAPK, or TAK1 pathways. Furthermore, TRIF recruits signaling complexes of noncanonical TBK1 (tank-binding-kinase 1) and IKKi (IKKε) to induce the phosphorylation of IRF3 (interferon regulatory factor 3) and IRF7, which translocated into the nucleus to induce the expression of type I IFNs (Fig. 3a).124
Role of TLRs in atherosclerosis
Dysregulation of TLRs is a key mechanism for inflammation and atherosclerosis, contributing to the development of cardiovascular diseases.125,126 Current human, animal, and epidemiological experiments demonstrate that chronic infectious diseases and related biological pathogens are involved in the progression of atherosclerosis. Accumulating evidence suggests that the expression of TLR4 and TLR2 increases in peripheral blood mononuclear cells, monocytes, ECs, and VSMCs in atherosclerotic disease. The agonists of TLRs are more likely to be endogenous factors. Highly immunogenic oxLDLs are associated with the upregulation of TLRs.127 Oxidized phospholipids in minimally modified LDL can bind and activate TLR4 on macrophages.128 Recent study revealed that oxLDL regulated the expression of TLR2 and TLR4 in cultured HUVECs.129 Furthermore, studies in mice have determined a central role for TLRs 1-9 in the development of atherosclerosis.130,131,132,133,134 Activation of TLR2 and TLR4 may play a profound role in infection-related atherosclerosis.135 TLR4 deficiency improved atherosclerosis in ApoE knockout (ApoE−/−) mice and LDL receptor-deficient (Ldlr−/−) mice,136,137 and markedly reduced atherosclerosis induced by oral bacteria. Subsequent follow-up research also confirmed that deletion of the shared TLRs signaling135 or adaptor including MyD88, TRAM, and TRIF leads to a reduction in plaque burden to varying degrees.138,139,140 However, it is controversial about the functions of TLR7 and TLR9 in the formation of atherosclerotic plaques.141 The hypothesis that pathogen-mediated TLR activation contributes to atherosclerosis remains to be demonstrated by more research.
Potential therapeutic targets
The roles of TLRs in inflammation promote the development of the therapeutic potential of targeting TLRs in the treatment of atherosclerosis. Animal experiments suggest that inhibiting TLR signals or blocking pathogen-associated molecular patterns (PAMPs) may be used for the treatment or prevention of atherosclerosis.142,143 Drugs both inhibiting the activity of intestinal bacterial LPS and blocking the TLR2- and 4-dependent signaling pathways can reduce the inflammation-activating pathways in atherosclerosis of mice and humans.144,145 Infection of Porphyromonas gingivalis accelerated atherosclerosis, which can be prevented via immunization in animal models.146 Ldlr−/− mice immunized with Streptococcus pneumoniae display an increase in the specific antibodies to oxLDL and decreased atherosclerotic lesion.147 However, multiple clinical trials revealed that anti-infective therapies are inefficacy in mitigating atherosclerotic diseases.148 Increasing evidence suggests that annual influenza vaccination reduces all-cause mortality of atherosclerotic patients, with no negative impact on recipients.135 Immunization with oxLDL or natural homologous LDL by vaccine formulations containing different adjuvants exerts atherosclerotic protection in animals.149 Therefore, vaccines against exogenous and endogenous antigens may represent a major translational goal for the treatment of atherosclerosis.
NLRP3 inflammasome
Inflammasomes are the complexes of multimeric cytosolic proteins and assemble in response to damage-associated molecular patterns and PAMPs, representing the inflammatory responses.150 The NLRP3 inflammasome (LRR, NACHT, and PYD domains-containing protein 3) that is well known, senses the endogenous danger signal activated by cholesterol crystals, activates caspase-1 to cleave pro-IL-1β and pro-IL-18 into mature and biologically active IL-1β and IL-18.122,151 The NLRP3 inflammasome is highly expressed in a variety of cell types, including innate immune cells and non-immune cells involved in the pathogenesis of the atherosclerotic cardiovascular disease.152,153,154
Structure of NLRP3 inflammasome
NLRP3, as a cytosolic protein, consists of an amino-terminal PYD (pyrin domain) that interacts with ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), a central NACHT domain (nucleotide-binding and oligomerization domain) that possesses ATPase activity, and a rare LRR (leucine-rich repeat) domain that can fold back onto the NACHT domain to induces autorepression.155 ASC, as an adapter protein, provides a bridge between NLRP3 and caspase-1. The PYD domain is also required for ASC self-association as well as interaction with NLRP3.156 Caspase-1 is initially synthesized as inactive zymogens and is produced by proteolytic cleavage.157
Activation of NLRP3 inflammasome
Canonical activation of NLRP3 inflammasome involves two hits: priming and activation. The priming step is induced by TLRs and cytokine receptors, such as TNF receptor or IL-1 receptor, and then NF-κB is activated and upregulates transcriptionally NLRP3 and pro-IL-1β, which promotes NLRP3 to form inflammasome assembly.158,159 The second step requires the activated NLRP3 oligomerization to recruit caspase-1 via ASC adaptor, ultimately leading to proteolytic cleavage of pro-IL-1β and pro-IL-18 into their active forms.122 Priming by oxLDL depends on binding of oxLDL to CD36 and the formation of CD36-TLR4-TLR6 complex, and internalization of the oxLDL results in lysosomal damage via activation of NLRP3.160 The P2X7 receptors are ligand-gated ion channels and can be opened by binding of extracellular ATP, which increases Na+ and Ca2+ influx and promote K+ efflux through coordinating with the K+ channel, leading to the activation of NLRP3 inflammasome.161
The noncanonical NLRP3 activation pathway is initiated by these caspases direct binding to intracellular LPS (iLPS) produced by Gram-negative bacteria, independent of TLR4, the conventional LPS receptor.162 For example, caspase-4, -5, and -11 can indirectly promote the mature pro-IL-1β and pro-IL-18 by activation of the NLRP3 inflammasome via binding to iLPS.163,164 Moreover, activated caspase-4, -5, and -11 induce pyroptosis via cleaving gasdermin D in a similar manner as caspase-1,165,166 and do not process directly pro-IL-1β and pro-IL-18.167 Caspase-11 induces production of extracellular ATP and in turn activates P2X7 receptor and promotes K+ efflux, leading to the activation of NLRP3 inflammasome and release of IL-1β,168 suggesting the link between noncanonical and canonical NLRP3 inflammasome pathways (Fig. 3b).169 In addition, the NLRP3 inflammasome is also activated by LPS via an alternative pathway that does not require K+ efflux, ASC speckle formation, or pyroptosis, which was only found in human monocytes.170
Regulation of NLRP3 inflammasome
The activation of NLRP3 inflammasome is tightly regulated by several innate immune molecules during infection and inflammation.171 The basal expression of NLRP3 is not typically sufficient for NLRP3 inflammasome activation. Numerous regulators promoting the NLRP3 inflammasome formation and activation have been identified, such as MyD88 and TRIF contributing to the priming of the NLRP3 inflammasome,172 caspase-8, and FADD (Fas-associated death domain protein) to mediate the priming and activation of the canonical and noncanonical NLRP3 inflammasome.173 In addition, the stress granule protein DDX3X binds to and activates NLRP3 inflammasome.174 In contrast, negative regulators of the NLRP3 inflammasome, such as the E3 ligase A20/TNF-α-induced protein 3 and TGF-β-activated kinase 1 (TAK1), prevent its excessive activation and suppress inflammation.175,176
In addition, the NLRP3 inflammasome is also regulated by cellular processes, such as ribosome stalling, translation inhibition,177 and post-translational modifications of NLRP3. Recent observation suggested that NEK7, a serine/threonine kinase, is required for activation of NLRP3 inflammasome via interaction with the nucleotide-binding domain and LRR of NLRP3.178 The post-translational modifications are critical for the priming of NLRP3 inflammasome activation. However, some of the post-translational modifications prevent NLRP3 inflammasome activation,169 or stabilize the NLRP3 in a signal-competent but the auto-suppressed state.179 The ubiquitination of NLRP3 mediated by E3 ligases and deubiquitinating enzymes regulate NLRP3 stability. E3 ubiquitin ligases that promote K-48 linked polyubiquitination, such as tripartite motif-containing 31, attenuate activation of NLRP3 inflammasome by proteasomal degradation.180 Additionally, NLRP3 was sumoylated at basal state via conjugating with small ubiquitin-like modifier (SUMO)-2/-3, which was mediated by MAPL/MUL1, a SUMO E3 ligase.181 The sumoylation of NLRP3 promotes oligomerization of ASC and activation of the inflammasome. SENP3 (SUMO-specific protease 3) mediates the NLRP3 desumoylation, leading to reduced ASC recruitment and NLRP3 inflammasome activation.182 Recent studies revealed the importance of the NLRP3 phosphorylation in the priming step.183 JNK1-mediated phosphorylation of NLRP3 at S194 is a key priming event, which is required for the NLRP3 activation. The phosphorylation at Ser295 of NLRP3 promotes NLRP3 oligomeric assembly.
Role of NLRP3 inflammasome in atherosclerosis
Previous clinical and experimental studies have demonstrated that IL-1β, as a pro-atherogenic cytokine, is involved in atherosclerosis progression, suggesting that NLRP3 inflammasome is presumably a key element in atherosclerotic pathogenesis.153,184 Indeed, the expression of NLRP3 inflammasome is increased in the plaques and peripheral blood mononuclear cells of atherosclerosis patients, which possibly reflected the severity of atherosclerosis. ASC knockout mice displayed reduced neointimal hyperplasia after injury, indicating the linking between the NLRP3 inflammasome and atherogenesis.185 Using bone marrow-transplanted mice provide further evidence that NLRP3 inflammasome participates in atherosclerotic progression. Loss of ASC, NLRP3, or IL-1 in bone marrow cells of Ldlr−/− mice ameliorated the atherosclerotic lesion.186 Deficiency of NLRP3 in Ldlr−/− mice seem to have merely small influences on atherogenesis.187 Other studies also showed that treatment with the selective NLRP3 inhibitor MCC950 or lentivirus-mediated NLRP3 silencing reduced atherosclerotic progression in ApoE−/− mice, further suggesting a causative role of NLRP3 inflammasome.188 However, other studies showed that there were no significant differences in macrophage infiltration or plaque size between ApoE−/− mice and ApoE−/− mice with deficient in ASC, NLRP3, or caspase-1, representing conflicting views that atherosclerosis progresses independently of the NLRP3 inflammasome in ApoE−/− mice.189 Possible explanations for this discrepancy are differences in the atherogenic diet, the hyperlipidaemia level, and even the sex-specific effects, which may affect host immune and inflammatory responses,153 as NLRP3 deficiency in bone marrow cells attenuated atherosclerotic lesion in female but not male Ldlr−/− mice.190
Most studies showed that monocytes promote phenotypic switching of VSMCs through activation of NLRP3 inflammasome, which exerted a likely detrimental role in the plaque stability in humans.154 In addition, mitochondrial dysfunction, oxidative stress, lysosome rupture, and endoplasmic reticulum (ER) stress as well as extracellular Ca2+, which are involved in activation of inflammasome, all existed in the plaques, especially in necrotic cores, and however, few studies have been conducted on these mechanisms in atherosclerosis.191
Potential therapeutic targets
Because of the pivotal role of NLRP3 inflammasome in the development of atherosclerosis, inhibiting NLRP3 inflammasome activation or the pharmacological inhibitors targeting NLRP3 inflammasome components that include P2X7 receptors antagonist, inhibition of caspase-1, and anti-IL-1, may have beneficial effects in protecting from inflammatory damage in atherosclerosis.192 To date, several small-molecule drugs targeting NLRP3 inflammasomes have been identified and employed in preclinical studies of cardiovascular inflammation.179 The synthetic small molecules, MCC950, CY-09, and OLT1177 bind directly to the NACHT domain of NLRP3 and block its ATPase activity.169 MCC950 that is the most representative inhibitor of the NLRP3 inflammasome activation, potently inhibits ATP-triggered, NLRP3-mediated IL-1β production,193 reduces macrophage infiltration and lesion size via attenuating inflammation and pyroptosis in hypercholesterolemia and hyperglycemia-induced atherosclerosis in mice.188,194,195 CY-09 directly interacts with the NACHT domain and disrupts ATP binding to NLRP3, which shows excellent preventive and therapeutic effects in mouse models.196 The small-molecule inhibitors VX-765, a caspase-1 inhibitor prodrug activated by intracellular esterases, can mitigate atherosclerosis in mice.197 For therapeutic purposes, the specificity of the potent target sites would be the critical prerequisite for the development of new inhibitors of NLRP3 inflammasome.
In recent years, NPs have been widely reported for the specific delivery of anti-inflammatory agents, peptides, antibodies, or small RNA directly on atherosclerotic lesions.70 For example, systemic delivery of methotrexate to macrophages via nanoconstructs has been shown to constitute an effective strategy for limiting the progression of atherosclerosis.198 Anti-miR33 nanotherapies significantly promoted reverse cholesterol transport and notably regulated adaptive immunity via modulating macrophage polarization and Tregs differentiation.199 Similar improved approach, applying the novel plug and play functionalized erythrocyte nanoplatform targeted drug delivery and acetic acid-control drug release show a marked improvement in atherosclerosis.200 In addition, based on the inherent affinity of macrophages for atherosclerotic lesions to construct biomimetic NPs fabricated with a macrophage membrane coating on the surface of rapamycin-loaded poly copolymer NPs has been demonstrated to effectively inhibit the progression of atherosclerosis.201 Although these findings suggest that using NPs to deliver anti-inflammatory substances is promising results, further basic research is necessary to understand more about the underlying functional mechanisms of the NPs. Other therapeutic strategies and current clinical trials targeting the NLRP3 inflammasome for atherosclerosis treatment would be described in the Atherosclerosis Therapies section of this review.
Proprotein convertase subtilisin/kexin type 9
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is now identified as an important and major player in the pathophysiology of atherosclerosis.202 PCSK9 is a soluble protein synthesized as a zymogen, and degrades LDL receptors (LDLR) upon activation by autocatalytic cleavage in the ER and subsequently increases LDL cholesterol (LDL-C) levels.
Expression of PCSK9
PCSK9 is mainly synthesized and secreted by the liver, and is also expressed in the central nervous system, lung, kidney, intestine, and blood vessel cells. The expression of PCSK9 is regulated by different transcription factors, including sterol-response element-binding proteins (SREBP) 1 and 2, peroxisome proliferator-activated receptor (PPAR) α and γ, as well as sirtuin (SIRT) 1 and 6. Among them, SREBP1 and SREBP2 have been well understood,203 which promote the expression of PCSK9 gene. In addition, PPARγ increases the PCSK9 gene expression in the liver,204 while peroxisome proliferator-activated receptor-α (PPARα),205 SIRT1, and SIRT6 decrease its expression.206 Pro-PCSK9 includes five domains: a signal peptide (aa 1–30), an N-terminal prodomain (aa 31–152) that is cleaved in the ER, a catalytic domain (aa 153–421) that contains the active sites Asp186, His226, and Ser386, a region containing 18 amino acids that links the catalytic domain to the C-terminal cys-his-rich domain (CHRD) (aa 440–692).207,208,209 Post-translational modifications are required for pro-PCSK9 maturation, in particular, autocatalytic cleavage of the prodomain of pro-PCSK9 in the ER. PCSK9 in vascular cells such as ECs, VSMCs, and macrophages can also be activated by pro-inflammatory cytokines and LPS.210,211,212
Role of PCSK9 in atherosclerosis
PCSK9 promotes hepatic LDLR lysosomal degradation, which has a pivotal role in cholesterol homeostasis and atherosclerosis. PCSK9 can also regulate LDLR levels in immune and vascular cells.213 PCSK9 secreted by VSMC and ECs downregulates LDLR expression on the surface of macrophages214 in a paracrine manner,215 and inhibits foam cell formation. Pcsk9−/− SMCs protected from neointimal hyperplasia via the reduced capacity of proliferation and migration.216
Beyond cholesterol metabolism, other physiological processes are also regulated by PCSK9, such as adipogenesis modulation, immune response, and interaction with many other cellular receptors, including oxLDL receptor-1 (LOX-1), VLDL receptor (VLDLR), and ApoE receptor 2 (ApoER2), CD36, and LDL receptor-like protein-1 (LRP-1).212 Some of these, including CD36 and LRP-1, are potent signaling receptors expressed on vascular and hematopoietic cells and thus PCSK9 might very well regulate important hemostatic systems, including inflammation, hemostasis, and tissue repair.217 PCSK9 in VSMCs exacerbates atherosclerotic lesion via self-reinforcing crosstalk of ROS/NF-κB/LOX-1/oxLDL axis activated by inflammation.202 PCSK9 induces LOX-1 expression and in turn, LOX-1 activation upregulates PCSK9 expression in VSMCs, which is a positive feedback regulation between the two.210 PCSK9 and LOX-1 share many cellular signaling pathways activating vascular inflammation and cellular apoptosis (Fig. 3c).
PCSK9 promotes the activation of platelet and thrombosis by interaction with platelet CD36.218 PCSK9 upregulates TLR4 expression and NF-κB activation to induce pro-inflammatory cytokine and tissue factor expression in a variety of tissues,219,220 and thus PCSK9 acts as a pro-inflammatory mediator. Several studies demonstrated that PCSK9 deficiency significantly reduced the levels of plasma pro-inflammatory cytokines IL-6, IL-8, TNF-α, and MCP-1.221 In addition, several epidemiological studies evaluated the association between PCSK9 and some key inflammatory markers including fibrinogen, hs-CRP (high-sensitivity C-reaction protein), and white blood cells, further suggesting the link between PCSK9 and inflammation.212 Importantly, the possible non-lipid-lowering effects of PCSK9, such as activation of platelets, the inflammatory burden of atherosclerosis, and metabolism of triglyceride-rich lipoprotein, have been identified and inhibition of PCSK9 acts as a safe potential therapeutic target to prevent thrombosis in patients who may be at higher risk because of elevated PCSK9 levels by hereditary or acquired factors as inflammation or using statins.217
Potential therapeutic targets
During the past decade, passive immunotherapy using PCSK9 monoclonal antibodies (mAbs) is an important breakthrough in lipid-lowering therapy. Various approaches, including mAbs, peptide inhibitors, or silencing PCSK9 mRNA expression and translation, have been identified to reduce the levels of plasma PCSK9.222,223,224 However, the studies of animal models showed that PCSK9 inhibitors offer a direct anti-inflammatory effect independent of the reduction in LDL-C, which needs further research.
Vaccination is an actively pursued new strategy for PCSK9 inhibition. PCSK9 vaccines AT04A and alirocumab decreased levels of serum cholesterol, reduced vascular and systemic inflammation, and limited atherosclerosis development.225,226 The PCSK9 vaccine Qβ-003 was recently reported to obviously decrease LDL-cholesterol and total cholesterol (TC) and to reduce the lesion size in ApoE−/− mice. In addition, infiltration of macrophage and expression of TNF-α and MCP-1 were obviously reduced in ApoE−/− mice administered the PCSK9Qβ-003 vaccine.227 Furthermore, the PCSK9Qβ-003 significantly increased the plaque stability and regulated cholesterol transport by upregulating the expression of LXR-α and ABCA1.
The specific property of a PCSK9 vaccine is able to induce the host to generate anti-PCSK9 antibodies that can disrupt the interaction between PCSK9 and LDLR.228 To this end, L-IFPTA (liposomal immunogenic fused PCSK9-tetanus peptide plus Alum adjuvant) is a recently designed novel PCSK9 vaccine.229 L-IFPTA vaccine contains a PCSK9 sequence as a B-cell epitope, and a T-helper cell epitope belonging to tetanus toxin proteins. So far, L-IFPTA vaccine has been shown to have long-lasting preventive and therapeutic effects on atherosclerosis in mice.230,231 The L-IFPTA vaccine also decreases the increased IFN-γ-inducing T-cell levels and obviously elevates Th2 cell levels and IL-4 and IL-10 expression in hypercholesterolaemic mice.228 Altogether, these studies identify the potential of L-IFPTA vaccine as a potent candidate for the treatment of dyslipidaemia and atherosclerotic disease.228 Other therapeutic strategies and current clinical trials targeting PCSK9 for atherosclerosis treatment would be described in the “Atherosclerosis therapies” section of this review.
Notch
The Notch is an evolutionarily conserved cellular signaling pathway that mediates intercellular communication and is involved in the regulation of most tissue development and homeostasis.
Core Notch pathway
Four isoforms of Notch receptors (Notch1–4) and five types of Notch ligands (Dll 1, 3, and 4, and Jagged1 and 2) are expressed in mammals.232 A single precursor of Notch receptors that is initially synthesized migrates to the Golgi apparatus and is cleaved by a furin-like protease into a membrane-distal and a membrane-anchored subunit. In the canonical Notch signaling, the Notch ligand binding to its receptor results in the removal of the extracellular fraction, and then two protein hydrolysis by A disintegrin and metalloprotease (ADAM10/17) and subsequently by a γ-secretase, respectively,233 results in the generation of the Notch active form, NICD (Notch intracellular domain). The NICD translocates into the nucleus to activate the transcription of target genes via binding to RBPJ (recombination signal-binding protein for immunoglobulin kappa J region) and other factors such as p300 and CBP-associated factor (PCAF). The Notch signaling also functions through “noncanonical” pathways. The activity of NICD occurs independently of the binding to canonical ligand or RBPJ and the γ-secretase cleavage.234 Noncanonical Notch signaling can interact with other pathways, including mTORC2 (mammalian target of rapamycin complex 2)/AKT, IκB kinase (IKK) α/β pathways,235 or Wnt/β-catenin. For example, noncanonical Notch pathway modulates mitochondrial function to promote cell survival by activation of mTORC2/AKT signaling with PINK1 (PTEN-induced kinase 1).236
Roles of Notch in atherosclerosis
Accumulating evidence has demonstrated that Notch protects from the dysfunctions of endothelium caused by inflammatory cytokines,237 and that Notch receptors mediate communication between ECs and VSMCs, and regulate the cell phenotypes.238,239,240 A growing number of studies show that the Notch plays a critical role in the transduction of the signals induced by flow shear stress to ECs.241 Activated Notch provides an anti-inflammatory, anti-atherogenic and pro-survival environment242,243 and maintains the endothelial integrity by mediating the formation of the tight junction complex in EC.244,245
Notch is also key signaling for regulating the structure and function of VSMCs. The expression of Notch receptors 2 and 3, and the main ligand Jagged1 are found in VSMCs. The mutation of Notch 2 and 3 can lead to defects in the development of VSMCs, which provides strong evidence for the implication of Notch signaling in regulating vascular differentiation and maturation during angiogenesis.246 Jagged1-Notch3 signaling mediates nidogen-2 to maintain the contractile phenotype of VSMCs through in vitro and in vivo.247 Although abundant studies have identified that Notch has a central role in the control of SMC development and function, and intimal repair, much less is known about its role in the fate of VSMCs in atherosclerotic development and progression. Recent studies demonstrated that Notch signaling is required for the adhesion of VSMCs but not other types of VSMC-derived cells in the formation of the cap in mouse atherosclerosis, and reduction of Notch signaling is a prerequisite for medial VSMC mediating the development of plaque,248 suggesting that sequential loss and gain of Notch signaling is required for the recruitment of cap SMC population in atherosclerosis.249
Notch regulates atherosclerosis by controlling the differentiation of macrophages into M1 or M2 subtypes.235 Notch1 induces M1 differentiation and enhances inflammatory responses by promoting MCP-1, IL-6, and TNF-α secretion. Instead, inhibition of Notch1 promotes differentiation of M2 macrophage and the production of the anti-inflammatory cytokines IL-1 receptor antagonist (IL-1Ra) and IL-10.250,251 It was shown that treatment with the DAPT, a Notch inhibitor, reduced macrophage migration and suppressed ICAM-1 expression in macrophages, thereby reducing macrophage infiltration in the plaques of ApoE−/− mice.252 Another study demonstrated that DAPT enhanced the anti-atherogenic activity of the LXR ligand agonist T317 in ApoE−/− mice while limiting the development of hypertriglyceridemia and fatty liver.253 In addition, Notch1 upregulates IL-6 expression in activated macrophages via the NF-κB pathway.254 IL-6 activates STAT3 (the signal transducer and activator of transcription-3), which in turn induces the expression of DLL1 and activates Notch signaling, establishing a positive feedback loop.255 DLL4-Notch1 axis promotes the polarization of M1 macrophages and blocks M2 polarization in the development of atherosclerosis. Overall, these studies suggest that Notch signaling could act as a target in different cell types to interfere with atherosclerosis progression. Therefore, further research is needed to better understand the variety and contradictory actions of Notch signaling, which may help in ameliorating and preventing atherosclerotic development.
Potential therapeutic targets
Increasing evidence suggests the aberrant regulation of Notch signaling in inflammatory diseases, and thus the ligands and receptors of Notch are expected to act as attractive therapeutic targets. In theory, the potential of targeting Notch signaling to modulate inflammation, as a new approach, is attractive, and however, their design and implementation are difficult, at least partly due to the broad involvement of Notch signaling in homeostatic and regenerative processes.256 The two main strategies targeting Notch have been identified, including GSIs (γ-secretase inhibitors) that can block the active NCID release to reduce the levels of active Notch, and mAbs that can either inhibit ligation upon cell contact or prevent proteolytic cleavage via stabilizing the NRR-region. GSIs have been shown to reduce atherosclerosis progression in ApoE−/− mice, which exert reduced ICAM-1 expression and total plaque areas.252
Compared with inhibition of pan-Notch, the use of mAbs is a more attractive approach, as mAbs are beneficial over conventional drugs in terms of specificity for the target, potency, and dosing frequency. IgG1 is the most perfect molecular scaffold to design mAb, as it has a long plasma half-life and the ability to mediate the function of a potent effector.257 However, The inherent traits of IgG1 structure must be considered for Notch-targeting. The mAb binding to effectors may lead to not only therapeutic efficacy but the also detrimental effect to tissue expressing Notch, which depends on both mAb target and indication.256 Animal studies show that mAbs targeting individual Notch ligands or receptors exert reduced intestinal toxicity, compared with that found by GSI treatment.258,259 Additional experimental data show the potential of targeting Notch pathway in atherosclerosis treatment. Blockade of DLL4-Notch signaling with an anti-DLL4 mAb decreased accumulation of macrophage, diminished calcification of plaque, reduced insulin resistance, and inhibited progression of atherosclerosis in Ldlr−/− mice.260 Since the Notch signaling is a potentially attractive target candidate in atherosclerosis, further research is needed to elucidate the distinct biological roles of Notch receptors and ligands in the plaque progression and to validate the individual potentials as novel therapeutic strategies.256
Wnt
The Wnt pathway participates in all different stages of atherosclerosis, from endothelial dysfunction to lipid deposit, and from initial inflammation to plaque formation.
Wnt family of proteins
Wnt proteins are a family of secreted lipid-modified glycoproteins. There are 19 different Wnt proteins that have been identified in humans. The proteins of the Frizzled family are the best characterized Wnt receptors,261 including 10 Frizzled isoforms in humans and animals. The Frizzled proteins, as unconventional G-protein-coupled receptors, contain a cysteine-rich extracellular domain (CRD), a seven-transmembrane spanning domain, and a cytoplasmic tail.262 Wnt ligands bind to the Frizzled receptors via interaction with the Frizzled CRD.263
Wnt signaling pathways
Wnt pathway builds a complex signaling regulatory network. Three intracellular pathways for Wnt signaling have been identified, which include the canonical or Wnt/β-catenin pathway that activates gene transcription through β-catenin, and the noncanonical Wnt/PCP (planar cell polarity) pathway that regulates cytoskeletal dynamics through activating JNK (C-Jun N-terminal kinase) by small G proteins; and Ca2+-dependent pathways (Wnt/Ca2+ pathway), which affects cellular adhesion and related gene expression through the release of intracellular Ca2+.264 The canonical Wnt/β-catenin signaling mediates the post-translational regulation of β-catenin that is a chief downstream effector. The β-catenin is degraded by multiprotein complex β-catenin destruction complex in the absence of Wnt ligands. During intracellular signaling, Wnt ligands bind to frizzled receptors and LRP 5 or 6 that are members of the LDLR gene family, leading to β-catenin translocating into the nucleus to trigger the transcription of target genes. In canonical Wnt signaling, Wnt ligands bind to several different receptors to promote a variety of cellular processes, depending on the presence of coreceptors and the formation of the receptor complexes.265 In addition, Wnt signaling is regulated by DKK (dickkopf) and Wnt ligand scavenging sFRP (secreted frizzled receptor protein) family.
Role of Wnt signaling in atherosclerosis
Beyond regulating cell proliferation and differentiation, the Wnt pathway also controls lipid homeostasis and storage.266 The activation of Wnt signaling is negatively correlated with the atherosclerotic severity. Involvement of Wnt in atherosclerosis was initially found in clinical patients carrying the Wnt co-receptor LRP6 mutation.266 These patients show increased LDL-C, triglycerides, and fasting glucose levels.267,268 Genetic experiments in mice suggest that the function loss of LRP6 is associated with coronary artery disease.267 Similarly, LRP5 also prevents atherosclerosis. Reduced serum levels of Wnt ligands are directly involved in the development of atherosclerotic disease.269
It has recently been shown that with lipid-lowering potentiates, activation of the Wnt pathway enhances IL-4 responsiveness in macrophages through the PGE2/STAT3 axis.270 Dickkopf-2 (DKK2), a negative regulator of Wnt/β-catenin, is involved in the activation of macrophages during atherosclerosis. Knockdown of DKK2 significantly reduces the expression levels of genes associated with M1-type macrophage polarization but upregulates markers of M2-type macrophage polarization and significantly attenuated foam cell formation. DKK2 knockdown activates the Wnt/β-catenin signaling by promoting the entry of β-catenin into the nucleus of macrophages, which leads to macrophage inactivation.271 Meanwhile, the Wnt/β-catenin signaling pathway was activated and DKK1 levels were downregulated under palmitic acid (PA) induction. Knockdown of cysteine-rich angiogenesis inducer (CCN1) could reduce the induction of EC inflammation and apoptosis by PA through inactivation of Wnt/β-catenin.272 Low stress activates Wnt/β-catenin and promotes endothelial mesenchymal transition (EndMT).273
Several studies suggest that Wnt1, Wnt2, and Wnt3a mediate a direct link between canonical Wnt signaling and VSMC proliferation in the neointimal formation of atherosclerosis.274 Upregulation of Wnt3a reduced the content of blood lipid, decreased the levels of inflammatory cytokines and oxidative stress, and increased plaque stability in ApoE−/− mice.275 Canonical Wnt signaling and LRP5 are also involved in the formation of macrophage foam cells in humans.276
Potential therapeutic targets
Sclerostin, an inhibitor of canonical Wnt pathway, has been identified to prevent atherosclerosis.265 Linking extracellular inhibitor of Wnt/β-catenin signaling sclerostin and DKK1 (Dickkopf-1) to carotid intima-media thickness is recently reported in heart failure with reduced ejection fraction. Furthermore, DKK2 silencing alleviated M1 but increased M2 macrophage expression, and protected against lipid loading by activation of Wnt/β-catenin signaling.271 Matrix protein R-spondin 2 inhibits activation of the canonical Wnt/β-catenin pathway and lymphangiogenesis, and the resulting suppression of cholesterol efflux from atherosclerotic arteries,277 suggesting a novel role of Wnt in arterial cholesterol efflux. A more recent study characterized a novel role of Wnt signaling in enucleated erythrocytes and uncovered that regulation of the red blood cell (RBC) cytoskeleton and erythrocyte survival was controlled by noncanonical Wnt signals.278 Treatment of erythrocytes with Wnt5 increased RBC survival and improved oxygen delivery while maintaining normal cell morphology.278 The ability of the Wnt pathway to increase RBC flexibility may prove essential in combatting hypoxia by ensuring that RBCs better traverse occluded and inflamed vessels and capillaries. Perhaps increased oxygen delivery through improved erythrocyte health may slow the development of unstable plaque formation or thrombotic release and reduce the risk of myocardial infarction or stroke in patients with advanced atherosclerosis.279
Atherosclerosis therapies
In view of atherosclerosis as the leading cause of death worldwide, prospective prevention and prompt treatment of atherosclerosis reduce the risk of developing its clinical manifestations. One of the critical causes of atherosclerosis is dyslipidemia. A number of therapeutic measures can reduce lipid risk factors for atherosclerosis. However, even when dyslipidemia is well controlled, risk factors from other sources remain. Therefore, it is required to address residual risk beyond lipids. Thus, we discuss the clinical trials that are currently underway for certain atherosclerotic processes, and the effects of quelling inflammation and atherosclerosis in the clinic (Table 1).
Lipid-lowering drugs
PCSK9 inhibitors
As a serine protease, PCSK9 binds to the LDL receptor and targets it for lysosomal degradation, which provides an additional pathway to control plasma LDL cholesterol levels. Therefore, PCSK9 inhibitors are new and highly effective functions in lowering lipids.280 Although statins have been widely used, PCSK9 inhibitors are still needed to be used in patients with a high level of cholesterol.281 Because a majority large number of patients receiving statins or in combination with ezetimibe still fail to achieve their therapeutic goals.282 This treatment dilemma is actually exacerbated by the increasing demands on LDL levels in patients with cardiovascular disease.280
Targeting PCSK9 is at least one step ahead of statins for early intervention in lipid metabolism. Especially if patients can not tolerate statins due to adverse side effects, PCSK9 inhibitors are an alternative. In addition, PCSK9 inhibitors have been confirmed to be effective and safe in the treatment of familial hypercholesterinaemia.283 In recent meta-analyses, PCSK9 inhibitors such as two fully human anti-PCSK9 mAbs (alirocumab, evolocumab) showed a significant reduction in cardiovascular events such as coronary revascularization, myocardial infarction, and ischemic stroke, but fail to exhibit a beneficial effect on cardiovascular mortality.284,285 Currently, lerodalcibep using a PCSK9-binding domain (adnectin) and human serum albumin forming a recombinant fusion protein is another protein-based strategy to inhibit PCSK9 and has shown promising efficacy in phase III testing. In recent years, new approaches to block PCSK9 synthesis include inclisiran, a double-stranded siRNA that specifically targets and induces PCSK9 mRNA degradation, inhibits translation, protein synthesis, and plasma levels of PCSK9, thereby reducing the LDL-C levels in plasma.286 In clinical trials, inclisiran treatment results in a reduction by ~50% in plasma LDL-C levels.287 In addition, anti-PCSK9 vaccines are much less expensive to produce than anti-PCSK9 antibodies or siRNAs that require regular dosing. An anti-PCSK9 vaccine called AT04A consists of homologous mouse and mature human PCSK9 protein N-terminal epitope peptides from amino acid residues 153–692, which is able to reduce plasma lipid levels and hinder the development of atherosclerosis.288 Phase I clinical trial has demonstrated that AT04A is safe and immunogenic and exhibits significant LDL-C-lowering activity, justifying further development.289
ANGPTL3 inhibitors
The liver-specific secretory protein ANGPTL3 (angiopoietin-like proteins 3) significantly decreases in atherosclerotic cardiovascular disease. Loss of function of ANGPTL3 related to the benefits of lipid-lowering makes ANGPTL3 be a very attractive pharmacological target. ANGPTL3 inhibits the activity of endothelial lipase and lipoproteins lipase (LPL), resulting in elevated LDL and triglyceride (TG).290 Currently, two mechanisms have been found to affect ANGPTL3. A mAb targeting ANGPTL3 called evinacumab was designed to decrease LDL-C, non-HDL-C, and TGs by increasing the activity of LPL and other associated metabolic enzymes. In patients with homozygous familial hypercholesterinaemia, evinocumab could reduce the level of plasma LDL from 20% to 90% and TG ~50%.291 On the other hand, ANGPTL3-specific antisense oligonucleotides (ASOs) could inhibit ANGPTL3 synthesis. In the healthy group, this ASO could dose-dependently decrease TG by ~30% to 60%.292 In addition, LDL and HDL were highly positively affected. Future, clinical trials are needed to further quantify the effects of ANGPTL3 inhibitors on atherosclerotic cardiovascular disease.
ACL inhibitor
ATP citrate lyase (ACL) belongs to a type of cytosolic enzyme that works upstream of HMG-CoA reductase.293 Bempedoic acid is a recently approved oral compound that inhibits cholesterol synthesis pathways like statins, resulting in an increase in LDL receptor density and decreased cholesterol synthesis by inhibiting ACL. ACL links carbohydrates energy metabolism to fatty acids production by catalyzing the synthesis of acetyl-CoA, the essential substrate of fatty acids and cholesterol.294 In a large phase III trial, except for the same effect as statins or ezetimibe, bempedoic acid also exhibits a significant effect on LDL lowering with no increase in adverse events compared with placebo.295,296,297 However, further clinical trials on hard endpoints such as cardiovascular mortality and ischemic events are still lacking to date.
MTP inhibitor
Microsomal triglyceride transfer protein (MTP) takes functions as a stimulator of VLDL particle assembly and secretion through transferring TGs to ApoB. Lomitapide, an MTP inhibitor that blocks the assembly of metabolic precursors of LDL particles, is approved to treat familial hypercholesterolemia. As VLDL eventually changes to form LDL over time, reducing VLDL production while also decreasing the levels of LDL-C.298 Though previous clinical studies of lomitapide suggested a high incidence of gastrointestinal distress, transaminases, and fatty liver, these complications were offset by a decreased risk of atherosclerotic cardiovascular disease and improved quality of life.299 However, the follow-up clinical studies determined that lomitapide not only reduced the level of LDL-C, but also resulted in a reduction in plasma HDL-C and ApoAI.300,301 Owing to the combined benefits and risks, lomitapide is currently only available as an adjunctive orphan therapy to treat homozygous familial hypercholesterolemia.
DGAT1 inhibitor
Diacylglycerol acyltransferase 1 (DGAT1) takes a pivotal function in lipid metabolism by catalyzing the last step in the TG synthesis pathway. Pradigastat, an orally administered DGAT1 inhibitor is another treatment modality. In phase I/II trials, six familial chylomicronaemia syndrome patients showed a dose-dependent reduction in TG of 41–70% over 21 days of treatment with only mild transient gastrointestinal adverse events in patients receiving dose-ranging pradigastat.302 A trial from 106 participants with overweight or obesity receiving multiple escalating doses, pradigastat decreased postprandial glucose, insulin, and TG excursions while increasing postprandial glucagon-like peptide-1 (GLP-1) levels.303 Gastrointestinal adverse reactions, including nausea and diarrhea, are significant at the dose of 10 mg; dietary fat reduction improves tolerability, but these still limit the development and widespread use of this drug.303
Apo inhibitors and mimetic peptides
Lipoprotein(a) [Lp(a)] composed of ApoB100 covalently bound to Apo(a), has been recognized as an independent risk factor and a pro-atherogenic potential for cardiovascular disease, while low Lp(a) levels are commonly associated with a reduced risk of cardiovascular disease.304 Preclinical studies established that ASO IONIS-APO(a)RX specifically targeted hepatic LPA mRNA to reduce the levels of plasma Lp(a).305 Similar to IONIS-APO(a)RX, the research group later developed IONIS-APO(a)-LRx (now called AKCEA-APO(a)-LRx) that exhibited specific and efficient targeting to the liver. In phase I/II clinical study, IONIS-APO(a)-LRx have exhibited a prominent dose-dependent decrease in mean levels of Lp(a) without major adverse events and other clinical findings. Recently, pelacarsen (also termed AKCEA-APO(a)-LRx or TQJ230), is undergoing a phase III clinical study that is designed to recruit at least 7500 patients with a history of symptomatic peripheral arterial disease, ischemic stroke, or myocardial infarction (NCT04023552).
Mipomersen is a novel type of second-generation antisense oligonucleotide and commonly accumulates in hepatocytes wherein it binds to ApoB mRNA and limits mRNA availability, resulting in a decrease in the production of ApoB and other lipoproteins that need ApoB including LDL, VLDL, and chylomicrons.298 A meta-analysis demonstrated that, unlike lomitapide, mipomersen could significantly reduce Lp(a) by 20–40%, non-HDL-C by 28%, LDL by 35–47% without influencing the level of HDL-C.306 Though numbers of clinical trials have suggested that mipomersen has efficacy in lowering ApoB-related lipoproteins in patients with different hypercholesterolemic phenotype, its approval is currently only available to treat homozygous familial hypercholesterolemia as an adjunctive orphan drug due to adverse effects such as flu-like symptoms and transaminitis.306,307,308
Similar to mipomersen, as a second-generation chimeric antisense inhibitor for ApoCIII, volanesorsen blocks protein synthesis as well as limits mRNA availability through binding to the ApoCIII mRNA. ApoCIII inhibits hepatic uptake of LPL and TG-rich particles, which have been recognized as high risks to result in hypertriglyceridemia.309 Volanesorsen Phase II study showed a significant reduction in TGs ~40–80% compared with placebo, either as monotherapy or in combination with fibrates/statins.310 Currently, volanersorsen has completed a phase III clinical trial for patients with familial chylomicronemia syndrome (NCT02658175). Considering that TG and ApoCIII are important factors inducing arteriosclerotic cardiovascular disease, volanesorsen is expected to improve overall cardiovascular outcomes in the future.
CSL-112 is a superior type of rHDL infusion therapy containing purified native human ApoAI from human plasma and phosphatidylcholine derived from soybeans.311 Numerous phase I/II clinical studies have determined that CSL-112 is able to increase ApoAI, pre-β HDL levels, cholesterol efflux capacity with good tolerance, with no evidence of liver toxicity.312,313,314 At present, CSL-112 is being studied in phase III clinical trial that is aimed to enroll not <17,000 volunteers to assess the endpoint of major adverse cardiovascular effects (MACEs) in adults having a history of acute myocardial infarction (NCT03473223).
ACP-501
Lecithin cholesterol acyltransferase (LCAT) is an enzyme in plasma that mainly catalyzes cholesterol esterification reaction during HDL formation. ACP-501 is a solution of recombinant human LCAT produced in CHO cells. It catalyzes free cholesterol to form cholesteryl esters with concomitant production of HDL-C.315 In a phase I clinical trial, ACP-501 was commonly associated with a dose-dependent increase in the level of HDL-C up to 42% and a decrease in cholesterol esters of ~22% in patients with low levels of HDL-C and atherosclerotic heart disease.316 The recent study involving patients with ST-elevation myocardial infarction has confirmed dose-related increases in HDL cholesterol esters, TC esters, and HDL-C in these patients.317 Although current clinical trial results of ACP-501 have shown benefits in LCAT deficient individuals, it still needs to demonstrate whether these benefits apply to other high-risk patients with dysfunctional and low HDL-C.
PPARα modulator
The PPARα agonists belong to ligand-activated transcriptional regulators that are involved in regulating lipid metabolism and expression of key apoproteins that influence the level of HDL and TG-rich lipoproteins, control cholesterol transport in macrophages and affect inflammation response.318 As a novel and specific PPARα modulator, Pemafibrate can maximize the beneficial effects and minimize the adverse effects of fibrates used currently. In vivo and in vitro experiments have confirmed that pemafibrate has a more potent effect on PPARα activation than previous fenofibrate. Recently, a randomized and double-blind phase II clinical study has demonstrated that pemafibrate has a greater effect on lowering TG and HDL-C levels than fenofibrate.319 Moreover, the incidence of adverse effects is lower than that of fenofibrate.
Acetyl-CoA carboxylase inhibitor
Gemcbene, as an inhibitor of acetyl-CoA carboxylase (ACC), is a dicarboxylic acid, which can reduce the production of hepatic triglycerides and cholesterol and enhance the clearance of VLDL cholesterol.320 Phase II clinical trials demonstrated that compared with placebo gemcbene could significantly reduce plasma LDL-C, VLDL-C, ApoCIII, TG, and CRP levels and obviously increased HDL-C levels.321 Gemcbene will now continue its phase II POC trial and assess the “End of Trial” outcomes jointly with the US Food and Drug Administration to plan the anticipated phase III clinical trial. Currently, as for patients with homozygous familial hypercholesterolemia, gemcabine may be a potentially valuable therapy because of lowering LDL-C independent of LDLR function.322
Icosapent ethyl
Icosapent ethyl produced by the esterification of high-purity eicosapentanoic acid, has been approved for treating severe hypertriglyceridemia and reducing the risk of cardiovascular disease in patients with high TG levels and pre-existing atherosclerotic cardiovascular disease exceeding maximum intensity statin treatment.323 Recent clinical trials suggested a reduction by approximately 25% in cardiovascular disease events, and an obvious return in low attenuation plaque volume.324 Therefore, icosapent ethyl may be a good addition for patients with elevated TG levels to reduce LDL-C.
Glucose-lowering drugs
GLP-1 receptor agonists
Glucagon-like peptide-1 (GLP-1) has been found to show incretin-like activity, leading to enhanced glucose-induced insulin secretion in normal and diabetic humans. These findings and subsequent evidence suggest that GLP-1 inhibits gastric emptying, food intake, and glucagon secretion supporting to development GLP-1 receptor agonists (GLP-1 RA) for the treatment of type 2 diabetes mellitus (T2DM).325 GLP-1 RAs mainly include exenatide, liraglutide, albiglutide, dulaglutide, lixisenatide, benaglutide, and semaglutide. GLP-1 receptor agonists, such as exenatide and lixisenatide were based on exendin structure and artificially synthesized, which showed a low homology to human GLP-1 and also had a resistant effect on the degradation of dipeptidyl peptidase-4 (DPP-4).
Lixisenatide is the first GLP receptor agonists to undergo extensive cardiovascular studies. Clinical trials showed that lixisenatide did not obviously alter the incidence of major cardiovascular events in >6000 patients with T2DM at risk for atherosclerosis.326 Therefore, lixisenatide did not differ from the placebo group in the rate of cardiovascular diseases in T2DM patients with high cardiovascular risk. Although the results regarding cardiovascular benefits are neutral, lixisenatide should not be discarded. Because the associated glycemic reduction may also decrease the long-term risk of cardiovascular and microvascular disease.327
Another class of GLP-1 receptor agonists is based on the natural human GLP-1 structure, which is processed by local modification of human GLP-1 molecular structure and has high homology to human GLP-1amino-acid sequences, such as liraglutide and benaglutide. The occurrence of MACEs, cardiovascular disease mortality, and total mortality was decreased in liraglutide-treated subjects. The incidence of microvascular disease, primarily reflecting a decrease in microalbuminuria events, was also lower in the liraglutide arm.328 Semaglutide, a GLP-1 RA structurally similar to liraglutide, was found to reduce MACEs occurrence which mainly reflected a reduction in the numbers of non-fatal strokes and myocardial infarction. A recent study indicates that semaglutide is associated with persistent, clinically relevant weight loss.329
The dulaglutide and cardiovascular outcomes in T2DM study showed that dulaglutide reduced the composite cardiovascular outcomes during 5-year period. Furthermore, liraglutide can be added to the management of patients with diabetes and other risk factors of cardiovascular diseases to decrease glucose concentrations, minimize hypoglycemia, decrease blood pressure and weight, and decrease the risk of cardiovascular diseases. However, the rate of gastrointestinal adverse events was higher than in patients using dulaglutide than other GLP-1 RAs.330
SGLT-2 inhibitors
Selective sodium-glucose co-transporter 2 inhibitors (SGLT2i) provide an insulin-independent mechanism to decrease glucose levels and have been approved as a therapeutic strategy for T2DM. They promote urinary glucose excretion (up to ~50%) by decreasing the reabsorption of glucose in the urine by the proximal tubules of the kidneys.331 Currently, findings show that despite only a modest decrease in glycated hemoglobin, SGLT-2 inhibitors improve cardiovascular outcomes in T2DM patients, with no conclusive evidence of additional safety issues.332 Researches suggested that SGLT2i exhibited a moderate benefit to decrease the risk of MACE, which appeared to be limited to patients with confirmed atherosclerotic cardiovascular disease. But, regardless of a history of heart failure or atherosclerotic cardiovascular disease, they have substantial benefits in reducing heart failure hospitalizations and kidney disease progression.333
Empagliflozin, ertugliflozin, dapagliflozin and canagliflozin were all belongs to SGLT2i. Ertugliflozin was demonstrated the non-inferiority versus placebo on MACE in patients with atherosclerotic cardiovascular disease and T2DM.334 Dapagliflozin treatment did not lead to lower or higher MACE compared to placebo in T2DM patients at risk for atherosclerotic cardiovascular disease rate but did decrease the rate of heart failure or cardiovascular death hospitalizations.335 Like other drugs in its class, empagliflozin has a lower inherent risk of hypoglycemia due to its insulin-independent mechanism of action, allowing it to be used as monotherapy and in combination with other anti-diabetic drugs with complementary modalities components to improve glycemic control efficiency in T2DM patients. In addition to lowering glucose levels, empagliflozin has beneficial effects on many non-glycemic outcomes, including modest reductions in blood pressure and body weight. As an adjunctive treatment to standard of care, it demonstrated cardioprotective and renoprotective properties in a mandatory cardiovascular outcome trial that was largely independent of glycemic control in patients with established cardiovascular disease and T2DM. Given its apparent cardioprotective effect, this drug may be worthy of prioritization among patients at high risk for cardiovascular events who require additional anti-diabetic drugs to achieve glycemic goals.336 As an SGLT2i, canagliflozin, decreases blood pressure, body weight, glycemia, and albuminuria in patients with diabetes. In two trials involving T2DM patients at high risk of cardiovascular disease, patients receiving canagliflozin had a lower risk for cardiovascular events than those receiving placebo, but a higher risk of amputation.337
DPP-4 inhibitors
Gliptins belong to a large class of anti-diabetic drugs inhibiting the activity of DPP-4, which is the major enzyme responsible for the degradation of GLP-1, thereby lowering blood glucose.338 Clinical trials suggested that DPP-4 inhibition could help hinder a variety of factors that lead to the development and progression of atherosclerosis, such as lowering lipid levels in plasma, inhibiting inflammatory response, and promoting vasodilation.339 In addition, the risk of hypoglycemia was lower when DPP-4 inhibitors were co-administered with sulfonylureas.340 Hypoglycemic episodes, especially severe episodes of hypoglycemia, are accompanied by an increased risk of major cardiovascular events and mortality in T2DM people.341 DPP-4 inhibitors, such as alogliptin, linagliptin, saxagliptin, sitagliptin, and vildagliptin have been widely available globally, which show a similar effect on reducing the risk of MACE. DPP-4 inhibition neither increases nor decreases the risk of the composite MACE outcome, including acute myocardial infarction, ischemic stroke, or cardiovascular death, with or without hospitalization for unstable angina in the composite endpoint. Therefore, the cardiovascular safety of these drugs has been demonstrated.341
The glycemic control of DPP-4 inhibitors varies by molecule, with monotherapy reducing glycated hemoglobin (HbA1c) by an average of 0.5–1.0%. Combination therapy with other anti-diabetic drugs may result in additional effects of HbA1c reduction.342 Alogliptin, linagliptin, saxagliptin, and sitagliptin have undergone a number of rigorous clinical studies in recent years, and the data of vildagliptin on safety and tolerability are available from various studies in meta-analyses obtained from a retrospective analysis.342
Linagliptin is one of the latest DPP-4 inhibitors in the gliptins market because it offers cardiovascular protection and renal safety.343,344,345 A large-scale clinical trial of linagliptin versus glimepiride on MACEs in T2DM patients concluded that among people with relatively early T2DM and elevated the risk of cardiovascular disease, receiving linagliptin led to a non-inferior risk for the composite cardiovascular outcome.346 Meanwhile, as for T2DM patients with renal and high cardiovascular risk, the addition of linagliptin to standard care led to a non-inferior risk for the composite cardiovascular outcome compared with placebo.347
The cardiovascular safety of sitagliptin was validated in trials evaluating cardiovascular outcomes with sitagliptin.348 Report showed that the risk of clinical adverse events did not increase during 18 weeks of sitagliptin treatment in patients in China, India, and South Korea.349 Notably, the major risk of cardiovascular events and hospitalization for heart failure have been assessed in diverse studies. Sitagliptin monotherapy did not increase morbidity/mortality and the risk of macrovascular/microvascular complications in T2DM.350 Meanwhile, long-term effects of sitagliptin on hospitalizations for heart failure were proven safe. A 3-year follow-up clinical study showed that sitagliptin did not increase the risk of hospitalization for heart failure or cardiovascular diseases.348 In addition, the incidence of in-hospital reinfarction, acute renal failure, and pulmonary edema were obviously decreased in patients with acute coronary syndrome and diabetes using sitagliptin.351,352 Sitagliptin showed even better results in reducing the rate of major cardiovascular events in patients with diabetes.353 Overall, sitagliptin is well tolerated as monotherapy or in combination with other common anti-diabetic drugs.
Targeting inflammatory pathways
Current treatments for atherosclerosis mainly focus on modulating traditional risk factors, including lipid-lowering and glucose-lowering drugs, to prevent and delay plaque formation and progression. Therapeutic drugs mainly include statins and anti-diabetic agents as well as metformin, which also exert anti-inflammatory effects. Anti-inflammatory interventions may represent novel potential therapies with additional beneficial effects in high-risk patients. We summarize the main therapeutic strategies in the following sections (Table 2).
Interleukin inhibitors
Experimental data on animals susceptible to atherosclerosis have confirmed that either pharmacological inhibition or genetic deletion of IL-1 signal pathways decreases atherosclerotic plaque area and progression rates. On the contrary, either increasing active IL-1 via injection of exogenous IL-1β, or reducing IL-1Ra, can result in plaque growth and exacerbation of atherosclerosis.354 Based on the above findings, the anti-inflammatory antithrombotic outcomes study of canakinumab aimed to demonstrate the role of the IL-1 signal pathway in atherosclerosis. Canakinumab (300 mg four times yearly) decreased the rates of recurrent MACE such as cardiovascular death, myocardial infarction, and ischemic stroke.355 Subsequent large-scale clinical trials firstly confirmed the efficacy of anti-inflammatory interventions in patients at risk for atherosclerotic events. The individual participants treated with canakinumab had a small but statistically significant increase in the risk of infection containing those with fatal outcomes. Furthermore, interventional treatments with canakinumab and IL-1Ra agonist (anakinra) demonstrated a key pathological role of IL-1β in the induction of inflammation-related diseases.356 These findings, therefore, provided evidence that inhibition of IL-1β could ameliorate the clinical outcome of cardiovascular disease.
The pleiotropic cytokine IL-6 plays a key role in numerous pathological processes, such as atherosclerosis and rheumatic diseases, which exhibits both pro- and anti-inflammatory properties depending on the targeted cell type. Ziltivekimab is a novel human IgG1, κ antibody directed against IL-6 ligand that has been previously investigated to examine the safety and pharmacodynamic effects in phase I/II trials of patients with rheumatoid arthritis (NCT01559103) and chronic kidney disease (NCT03126318). A recent study supported that ziltivekimab could cause a decrease in overall cardiovascular risk in patients with increased inflammation on hemodialysis by reducing inflammation.357
Tocilizumab and sarilumab, both mAbs against IL-6R, have been approved for combination used with other types of antirheumatic agents or as monotherapy in patients with rheumatoid arthritis and other inflammation-related diseases.358 Tocilizumab and sarilumab were tested in clinical trials for their efficacy as rheumatoid arthritis treatments among patients with cardiovascular disease.359 Though tocilizumab significantly perturbs cholesterol and lipid homeostasis, including increasing TC and LDL, it also shows beneficial effects on surrogate markers of cardiovascular risk. Other clinical studies of large numbers of patients with rheumatoid arthritis using tocilizumab in the postmarketing setting exhibit a lower rate of MACE.360
Methotrexate and folic acid are analogs of similar chemical structures. Preclinical studies have confirmed that methotrexate inhibits lymphocyte proliferation and inflammatory cytokines production via adenosine binding with A2 receptor, which is one of the most important anti-inflammatory mechanisms.361 Previous systematic reviews and meta-analyses suggested the significant relationships between methotrexate treatment and a reduced risk of cardiovascular disease.362 Similarly, a recent study of patients with rheumatoid arthritis showed that methotrexate use is associated with lower cardiovascular outcomes.363
NLRP3 inflammasome inhibitors
The NLRP3 inflammasome takes an important function in proteolysis and IL-1β maturation. As an essential sensor, functions in pathological processes are related to vascular endothelial dysfunction.364 Thus, targeting NLRP3 may be an effective and potential target for therapeutic intervention in acute and chronic cardiovascular disease. Colchicine, a natural product extracted from the bulb of autumn colchicum, has been successfully used in the treatment of inflammatory diseases for millennia. In the field of cardiovascular research, a number of studies have determined the effect of colchicine in decreasing recurrent cardiovascular events in atherosclerotic patients.365 Likewise, the low-dose colchicine trial-2 trial in patients with the chronic phase of coronary artery disease suggested similar efficacy.366
Dapansutrile, a specific NLRP3 inflammasome inhibitor, phase I randomized trial has demonstrated its safety and tolerability in heart failure patients with reduced left ventricular ejection fraction.367 According to this preliminary data, a dose of 2000 mg/d dapansutrile appears to be the possible dose to be tested in future studies. However, dapanusutrile appears no significant effect on C-reactive protein levels, the preferred inflammatory biomarker for cardiovascular risk stratification, and IL-1 itself is downstream of the activated NLRP3 inflammasome.368
p38 MAPK inhibitor
p38 MAPK is activated by multiple factors in the cardiovascular system, including oxLDL, hypertension, ischemia, and volume overload.369 Losmapimod is an oral, selective, and reversible competitive p38 inhibitor targeting MAPK activation. Early studies indicated the pharmacodynamic and pharmacokinetic basis of losmapimod in healthy and positive data on the safety and tolerability of losmapimod. However, in the phase III trial, using losmapimod treated acute myocardial infarction patients did not show a relationship with a decreased risk of the MACE.370 Notably, a disadvantage of the trial was the limited treatment course, whereby we could not exclude the possibility that lomatimod may be effective as an anti-inflammatory treatment for a longer duration.371
P-selectin antagonist
Inclacumab directly targets the cell adhesion molecule P-selectin, taking functions at the interface of thrombosis and inflammation. Inclacumab has appeared to significantly reduce myocardial damage in certain types of acute and chronic myocardial infarction.372 In addition, the P-selectin has been identified as the independent risk factor for peripheral arterial disease and decreased ankle-brachial index.373 Though the basic principle indicates that P-selectin antagonist inclacumab is able to improve the development of peripheral artery disease, evidence from clinical studies is lacking.374
Lp-PLA2 and sPLA2 inhibitors
The level and activity of lipoprotein-associated phospholipase A2 (Lp-PLA2) and secretory phospholipase A2 (sPLA2) in circulating blood have been confirmed to be biomarkers of increased risk of atherosclerotic cardiovascular disease.375 Two potent inhibitors against PLA2, darapladib (Lp-PLA2 inhibitor) and varespladib (sPLA2 inhibitor), have been investigated extensively in clinical studies. Phase III clinical trial on darapladib against ischemic events in stable coronary heart diseases showed that darapladib could not obviously decrease the risk of the primary composite endpoint of myocardial infarction, stroke, or cardiovascular death (NCT00799903). Similarly, a double-blind, randomized, multicenter trial also suggested that varespladib could not decrease the risk of recurrent cardiovascular events, but obviously increased the risk of myocardial infarction (NCT01130246). These findings indicate that besides serving as an inflammatory biomarker of cardiovascular disease deeper knowledge on PLA2 is needed.376
Succinobucol
Succinobucol is a novel type of phenolic antioxidant small molecule that is not easily degraded or modified by body metabolism, thereby inhibiting the formation of the hepatotoxic molecule spiroquinone and exhibiting an anti-inflammatory effect by binding to VCAM-1.377 In the phase III trial, 6144 patients at risk of cardiovascular disease were randomly divided into succinobucol and placebo group. Regretfully, the primary composite endpoint of myocardial infarction, unstable angina, cardiovascular death, resuscitated cardiac arrest, coronary revascularization or stroke did not decrease. However, the risk of key secondary endpoint was decreased by 19%, and patients’ risk of new-onset diabetes was decreased by 64%. Based on these, further studies may be required to elucidate the clinical effect of succinobucol on cardiovascular events in the future.
Conclusion and remarks
Atherosclerosis is a chronic inflammatory vascular disease primarily triggered by vascular cells and immune cells. In recent decades, inflammatory mediators, as potential common risk factors, have received extensive attention, which influences LDL and triglyceride-rich lipoprotein levels, and alters artery wall cell behaviors to beckon leukocytes. Rather than replacing traditional drivers like LDL, the concept of inflammation as a driver of atherosclerosis provides more mechanisms by which the traditional risk factors induce atherosclerotic disease and its complication. The development of single-cell analysis technologies provides deeper insights into the roles of various cells involved in atherosclerotic lesions. The contribution of VSMCs to atherosclerotic plaque development is largely underestimated previously.1,33 Advances in lineage tracing provide an available access route to re-evaluate this misdiagnosing situation. However, many questions still remain to be answered. For example, what are the roles of different VSMC phenotypes; how are the processes of VSMC phenotypic switching and transdifferentiation to other type-like phenotypes such as macrophage-like or myofibroblast-like connected. Though we understand some of the molecular mechanisms that regulate phenotypic switching of VSMCs, and a positive, negative or neutral effect of particular VSMC phenotypes on atherosclerosis and plaque stability, we currently lack experimental evidence in vivo from animal research or correlated data in human studies. Various immune cells are accumulated within complicated plaques, propagating the development of atherosclerosis and its complications. Recent reports highlight the complexity of the origins of lesion macrophages as well as the plasticity of these cells and uncover the specific characteristics of immune cell dysregulation within atherosclerotic plaques that give rise to clinical cardiovascular events, representing that identification of specific dysregulations of immune cells in the plaques associated with cardiovascular events may be required for the development of more precise immunotherapies of atherosclerosis.
A growing body of evidence demonstrates that the NLRP3 inflammasome and TLRs contribute to the development of atherosclerosis, and are identified to be a promising new target for the treatment of these diseases. Given the intimate link between dysregulation of NLRP3 inflammasome and atherosclerosis, it is both challenging and intriguing to reveal the complex mechanisms of regulation and activation of NLRP3 inflammasome and their influence on human health and disease. In addition, studies on the role of PCSK9 in different cells involved in at all stages of the plaque have supported that the effect of PCSK9 on atherosclerotic development is beyond the LDLR degradation, and occurs by crosstalking with other molecular mechanisms ranging from the activation of inflammatory pathways to apoptosis of ECs and migration of VSMCs. Therefore, it is needed to deeply understand the roles of PCSK9 in atherosclerosis by unveiling the precise molecular mechanisms underlying it.
Inflammation has opened the door to more new therapeutic targets for atherosclerosis treatment. In the last years, three clinical trials have translated this large body of genetic evidence and human biomarker and experimental work to clinical fruition.355,366,378 Indeed, the recent evidence that interventions of anti-inflammation can forestall the complications of atherosclerosis scratches merely the surface of the potential as novel therapeutics. NLRP3 inflammasome and the downstream pro-inflammatory cytokines IL-1β and IL-18 are attractive pharmaceutical candidates for therapeutic intervention of atherosclerosis, and selectively neutralizing these cytokines has been shown to be available in the context. Macrophage-targeting NPs can deliver a broad range of therapeutic drugs to atherosclerotic lesions, which suppresses pro-atherogenic processes of macrophages, promoting inflammation resolution and plaques stabilization.71 The challenge facing us is how to enhance beneficial efficiency, as interference with inflammatory signalings can impair host defenses. It is likely that the advances in multiple omics technologies will help to solve the multidimensional problem caused by the plentiful number of mediators and myriad of pathways that are susceptible to therapeutic intervention. Although translating basic findings to clinical treatment is challenging, increasing understanding of inflammation in atherosclerosis facilitated by recent technical advances provides optimism. Therefore, further work remains to be performed in harnessing the inflammatory concept to ameliorate and forestall atherosclerosis and its complications. Many unanswered questions in this field provide opportunities for future research and may yield therapeutic strategies to improve patient outcomes.
References
Basatemur, G. L. et al. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 16, 727–744 (2019).
Soehnlein, O. & Libby, P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat. Rev. Drug Discov. 20, 589–610 (2021).
Roy, P., Orecchioni, M. & Ley, K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat. Rev. Immunol. (2021). [Online ahead of print]
Hansson, G. K. & Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 (2011).
Ilatovskaya, D. V., Halade, G. V. & DeLeon-Pennell, K. Y. Adaptive immunity-driven inflammation and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 317, H1254–H1257 (2019).
Jaipersad, A. S., Lip, G. Y., Silverman, S. & Shantsila, E. The role of monocytes in angiogenesis and atherosclerosis. J. Am. Coll. Cardiol. 63, 1–11 (2014).
Ross, R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Wolf, D. & Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 124, 315–327 (2019).
Miller, Y. I. et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108, 235–248 (2011).
Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 (2010).
Fernandez, D. M. et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25, 1576–1588 (2019).
Bornfeldt, K. E., Linton, M. F., Fisher, E. A. & Guyton, J. R. JCL roundtable: lipids and inflammation in atherosclerosis. J. Clin. Lipido. 15, 3–17 (2021).
Mauricio, D., Castelblanco, E. & Alonso, N. Cholesterol and Inflammation in atherosclerosis: an immune-metabolic hypothesis. Nutrients 12, 2444 (2020).
Libby, P. Inflammation in atherosclerosis-no longer a theory. Clin. Chem. 67, 131–142 (2021).
Jinagal, J. & Dhiman, P. Retraction: retinal hemorrhage from blunt ocular trauma. N. Engl. J. Med. 382, 490 (2019).
Clark, B. C. & Arnold, W. D. Strategies to prevent serious fall injuries: a commentary on bhasin et al. a randomized trial of a multifactorial strategy to prevent serious fall injuries. Adv. Geriatr. Med. Res. 3, e210002 (2021).
Shao, C., Wang, J., Tian, J. & Tang, Y. D. Coronary artery disease: from mechanism to clinical practice. Adv. Exp. Med. Biol. 1177, 1–36 (2020).
Gao, Y. & Galis, Z. S. Exploring the role of endothelial cell resilience in cardiovascular health and disease. Arterioscler. Thromb. Vasc. Biol. 41, 179–185 (2021).
Lei, W. et al. MARCH5 restores endothelial cell function against ischaemic/hypoxia injury via Akt/eNOS pathway. J. Cell. Mol. Med. 25, 3182–3193 (2021).
Siragusa, M. et al. VE-PTP inhibition elicits eNOS phosphorylation to blunt endothelial dysfunction and hypertension in diabetes. Cardiovasc. Res. 117, 1546–1556 (2021).
Tajadura, V. et al. beta-catenin promotes endothelial survival by regulating eNOS activity and flow-dependent anti-apoptotic gene expression. Cell Death Dis. 11, 493 (2020).
Raina, P. et al. Association of eNOS and MCP-1 genetic variants with type 2 diabetes and diabetic nephropathy susceptibility: a case-control and meta-analysis study. Biochem Genet 59, 966–996 (2021).
Dobnikar, L. et al. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat. Commun. 9, 4567 (2018).
Kaur, H. et al. Single-cell profiling reveals heterogeneity and functional patterning of GPCR expression in the vascular system. Nat. Commun. 8, 15700 (2017).
Kapustin, A. N. et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res 116, 1312–1323 (2015).
Furmanik, M. et al. Endoplasmic reticulum stress mediates vascular smooth muscle cell calcification via increased release of Grp78 (glucose-regulated protein, 78 kDa)-loaded extracellular vesicles. Arterioscler. Thromb. Vasc. Biol. 41, 898–914 (2021).
Owens, A. P. 3rd et al. Angiotensin II induces a region-specific hyperplasia of the ascending aorta through regulation of inhibitor of differentiation 3. Circ. Res. 106, 611–619 (2010).
Alencar, G. F. et al. Stem cell pluripotency genes Klf4 and Oct4 regulate complex SMC phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation 142, 2045–2059 (2020).
Hong, X. et al. Transdifferentiated Human Vascular Smooth Muscle Cells are a New Potential Cell Source for Endothelial Regeneration. Sci. Rep. 7, 5590 (2017).
Davies, J. D. et al. Adipocytic differentiation and liver x receptor pathways regulate the accumulation of triacylglycerols in human vascular smooth muscle cells. J. Biol. Chem. 280, 3911–3919 (2005).
Pan, H. et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 142, 2060–2075 (2020).
Han, M. et al. Serum deprivation results in redifferentiation of human umbilical vascular smooth muscle cells. Am. J. Physiol. Cell. Physiol. 291, C50–C58 (2006).
Grootaert, M. O. J. & Bennett, M. R. Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc. Res. 117, 2326–2339 (2021).
Vengrenyuk, Y. et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler. Thromb. Vasc. Biol. 35, 535–546 (2015).
Shankman, L. S. et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21, 628–637 (2015).
Mattila, P. K., Batista, F. D. & Treanor, B. Dynamics of the actin cytoskeleton mediates receptor cross talk: an emerging concept in tuning receptor signaling. J. Cell Biol. 212, 267–280 (2016).
Solway, J. et al. Structure and expression of a smooth muscle cell-specific gene, SM22 alpha. J. Biol. Chem. 270, 13460–13469 (1995).
Han, M. et al. Smooth muscle 22 alpha maintains the differentiated phenotype of vascular smooth muscle cells by inducing filamentous actin bundling. Life Sci. 84, 394–401 (2009).
Liu, R., Hossain, M. M., Chen, X. & Jin, J. P. Mechanoregulation of SM22alpha/transgelin. Biochemistry 56, 5526–5538 (2017).
Xie, X. L. et al. Smooth muscle 22alpha facilitates angiotensin II-induced signaling and vascular contraction. J. Mol. Med. 93, 547–558 (2015).
Shu, Y. N. et al. CKII-SIRT1-SM22alpha loop evokes a self-limited inflammatory response in vascular smooth muscle cells. Cardiovasc. Res. 113, 1198–1207 (2017).
Shu, Y. N. et al. SM22alpha inhibits vascular inflammation via stabilization of IkappaBalpha in vascular smooth muscle cells. J. Mol. Cell Cardiol. 84, 191–199 (2015).
Hu, D. et al. Vascular smooth muscle cells contribute to atherosclerosis immunity. Front. Immunol. 10, 1101 (2019).
Lv, P. et al. SM22alpha loss contributes to apoptosis of vascular smooth muscle cells via macrophage-derived circRasGEF1B. Oxid. Med Cell Longev. 2021, 5564884 (2021).
Zhong, L. et al. SM22alpha (smooth Muscle 22alpha) prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching through suppressing reactive oxygen species/nf-kappab (nuclear factor-kappaB). Arterioscler. Thromb. Vasc. Biol. 39, e10–e25 (2019).
Chen, R. et al. Transcriptome profiling reveals that the SM22alpha-regulated molecular pathways contribute to vascular pathology. J. Mol. Cell Cardiol. 72, 263–272 (2014).
Dong, L. H. et al. Blockade of the Ras-extracellular signal-regulated kinase 1/2 pathway is involved in smooth muscle 22 alpha-mediated suppression of vascular smooth muscle cell proliferation and neointima hyperplasia. Arterioscler. Thromb. Vasc. Biol. 30, 683–691 (2010).
Lv, P. et al. SM22alpha inhibits lamellipodium formation and migration via Ras-Arp2/3 signaling in synthetic VSMCs. Am. J. Physiol. Cell Physiol. 311, C758–C767 (2016).
Lv, P. et al. Phosphorylation of smooth muscle 22alpha facilitates angiotensin II-induced ROS production via activation of the PKCdelta-P47phox axis through release of PKCdelta and actin dynamics and is associated with hypertrophy and hyperplasia of vascular smooth muscle cells in vitro and in vivo. Circ. Res. 111, 697–707 (2012).
Zhao, L. L. et al. Insulin-independent GLUT4 translocation in proliferative vascular smooth muscle cells involves SM22alpha. J. Mol. Med. 95, 181–192 (2017).
Dong, L. H. et al. TRAF6-mediated SM22alpha K21 ubiquitination promotes G6PD activation and NADPH production, contributing to GSH homeostasis and VSMC survival in vitro and in vivo. Circ. Res 117, 684–694 (2015).
Zhang, D. D. et al. Smooth muscle 22 alpha protein inhibits VSMC foam cell formation by supporting normal LXRalpha signaling, ameliorating atherosclerosis. Cell Death Dis. 12, 982 (2021).
Miao, S. B. et al. Accumulation of smooth muscle 22alpha protein accelerates senescence of vascular smooth muscle cells via stabilization of p53 In Vitro and In Vivo. Arterioscler. Thromb. Vasc. Biol. 37, 1849–1859 (2017).
Serpa, P. B. S. & Santos, A. P. Incidental diagnosis of a spindle cell type gastrointestinal stromal tumor in a dog with ethylene glycol intoxication. Vet. Clin. Pathol. 50, 70–75 (2021).
Richardson, A., Ganz, O. & Vallone, D. The cigar ambassador: how Snoop Dogg uses instagram to promote tobacco use. Tob. Control 23, 79–80 (2014).
Tinajero, M. G. & Gotlieb, A. I. Recent developments in vascular adventitial pathobiology: the dynamic adventitia as a complex regulator of vascular disease. Am. J. Pathol. 190, 520–534 (2020).
Lordan, R., Tsoupras, A. & Zabetakis, I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 45, 100694 (2021).
Ed Rainger, G. et al. The role of platelets in the recruitment of leukocytes during vascular disease. Platelets 26, 507–520 (2015).
van der Pol, E. et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharm. Rev. 64, 676–705 (2012).
Lee, M. K. S. et al. Apoptotic ablation of platelets reduces atherosclerosis in mice with diabetes. Arterioscler. Thromb. Vasc. Biol. 41, 1167–1178 (2021).
Theofilis, P. et al. Inflammatory mediators of platelet activation: focus on atherosclerosis and COVID-19. Int. J. Mol. Sci. 22, 11170 (2021).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Barrett, T. J. Macrophages in atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 40, 20–33 (2020).
Khoury, M. K., Yang, H. & Liu, B. Macrophage biology in cardiovascular diseases. Arterioscler. Thromb. Vasc. Biol. 41, e77–e81 (2021).
Gerlach, B. D. et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 33, 2445–2463 e8 (2021).
Boyle, J. J. et al. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ. Res. 110, 20–33 (2012).
Maretti-Mira, A. C. et al. Cholesterol-induced M4-like macrophages recruit neutrophils and induce NETosis. Front. Immunol. 12, 671073 (2021).
Yin, C. et al. Efferocytic defects in early atherosclerosis are driven by GATA2 overexpression in macrophages. Front. Immunol. 11, 594136 (2020).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
X. Huang, et al. Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages. Nat. Protoc. (2022). [Online ahead of print].
W. Chen, et al. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. (2021). [Online ahead of print].
Tao, W. et al. siRNA nanoparticles targeting CaMKIIgamma in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 12, 1063 (2020).
Yurdagul, A. Jr et al. ODC (ornithine decarboxylase)-dependent putrescine synthesis maintains MerTK (MER tyrosine-protein kinase) expression to drive resolution. Arterioscler. Thromb. Vasc. Biol. 41, e144–e159 (2021).
Dworacka, M. et al. Pro-atherogenic alterations in T-lymphocyte subpopulations related to acute hyperglycaemia in type 2 diabetic patients. Circ. J. 71, 962–967 (2007).
Nunez, J. et al. Low lymphocyte count and cardiovascular diseases. Curr. Med. Chem. 18, 3226–3233 (2011).
Tyrrell, D. J. & Goldstein, D. R. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 18, 58–68 (2021).
Sekiya, T. & Yoshimura, A. In vitro Th differentiation protocol. Methods Mol. Biol. 1344, 183–191 (2016).
Buono, C. et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl. Acad. Sci. USA 102, 1596–1601 (2005).
Wigren, M., Nilsson, J. & Kolbus, D. Lymphocytes in atherosclerosis. Clin. Chim. Acta 413, 1562–1568 (2012).
Saigusa, R., Winkels, H. & Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 17, 387–401 (2020).
Milner, J. D., Sandler, N. G. & Douek, D. C. Th17 cells, Job’s syndrome and HIV: opportunities for bacterial and fungal infections. Curr. Opin. HIV AIDS 5, 179–183 (2010).
Smith, E. et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121, 1746–1755 (2010).
Madhur, M. S. et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31, 1565–1572 (2011).
Taleb, S. et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 206, 2067–2077 (2009).
Veillard, N. R. et al. Differential expression patterns of proinflammatory and antiinflammatory mediators during atherogenesis in mice. Arterioscler. Thromb. Vasc. Biol. 24, 2339–2344 (2004).
Ley, K. Role of the adaptive immune system in atherosclerosis. Biochem. Soc. Trans. 48, 2273–2281 (2020).
Bonacina, F. et al. Adoptive transfer of CX3CR1 transduced-T regulatory cells improves homing to the atherosclerotic plaques and dampens atherosclerosis progression. Cardiovasc. Res. 117, 2069–2082 (2021).
Xia, M., Wu, Q., Chen, P. & Qian, C. Regulatory T cell-related gene biomarkers in the deterioration of atherosclerosis. Front. Cardiovasc. Med. 8, 661709 (2021).
Winkels, H. & Wolf, D. Heterogeneity of T cells in atherosclerosis defined by single-cell RNA-sequencing and cytometry by time of flight. Arterioscler. Thromb. Vasc. Biol. 41, 549–563 (2021).
Schafer, S. & Zernecke, A. CD8(+) T cells in atherosclerosis. Cells 10, 37 (2020).
van Duijn, J. et al. CD8+ T-cells contribute to lesion stabilization in advanced atherosclerosis by limiting macrophage content and CD4+ T-cell responses. Cardiovasc. Res. 115, 729–738 (2019).
Amirfakhryan, H. Vaccination against atherosclerosis: an overview. Hellenic J. Cardiol. 61, 78–91 (2020).
Back, M., Yurdagul, A. Jr, Tabas, I., Oorni, K. & Kovanen, P. T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16, 389–406 (2019).
Aguilar-Ballester, M. et al. Impact of cholesterol metabolism in immune cell function and atherosclerosis. Nutrients 12, 2021 (2020).
Tsiantoulas, D., Sage, A. P., Mallat, Z. & Binder, C. J. Targeting B cells in atherosclerosis: closing the gap from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 35, 296–302 (2015).
Mangge, H. et al. Beyond macrophages and T cells: B cells and immunoglobulins determine the fate of the atherosclerotic plaque. Int. J. Mol. Sci. 21, 4082 (2020).
Fillatreau, S. et al. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3, 944–950 (2002).
Blair, P. A. et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 32, 129–140 (2010).
Rincon-Arevalo, H. et al. Low frequency of IL-10(+) B cells in patients with atherosclerosis is related with inflammatory condition. Heliyon 6, e03441 (2020).
Kyaw, T. et al. Alarmin-activated B cells accelerate murine atherosclerosis after myocardial infarction via plasma cell-immunoglobulin-dependent mechanisms. Eur. Heart J. 42, 938–947 (2021).
Quillard, T. et al. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur. Heart J. 36, 1394–1404 (2015).
Franck, G. et al. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ. Res. 121, 31–42 (2017).
Pieterse, E. et al. Neutrophils discriminate between lipopolysaccharides of different bacterial sources and selectively release neutrophil extracellular traps. Front. Immunol. 7, 484 (2016).
Nahrendorf, M. & Swirski, F. K. Immunology. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science 349, 237–238 (2015).
T. Josefs, et al. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight. 5, e134796 (2020).
Doring, Y., Libby, P. & Soehnlein, O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ. Res. 126, 1228–1241 (2020).
Schumski, A. et al. Endotoxinemia accelerates atherosclerosis through electrostatic charge-mediated monocyte adhesion. Circulation 143, 254–266 (2021).
Hermans, M., Lennep, J. R. V., van Daele, P. & Bot, I. Mast cells in cardiovascular disease: from bench to bedside. Int. J. Mol. Sci. 20, 3395 (2019).
Kouhpeikar, H. et al. The effect of statins through mast cells in the pathophysiology of atherosclerosis: a review. Curr. Atheroscler. Rep. 22, 19 (2020).
Lee, M. et al. Mast cell chymase degrades apoE and apoA-II in apoA-I-knockout mouse plasma and reduces its ability to promote cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 22, 1475–1481 (2002).
Dounousi, E. et al. The innate immune system and cardiovascular disease in ESKD: monocytes and natural killer cells. Curr. Vasc. Pharm. 19, 63–76 (2021).
Le Bouteiller, P. et al. CD160: a unique activating NK cell receptor. Immunol. Lett. 138, 93–96 (2011).
Bonaccorsi, I. et al. Symptomatic carotid atherosclerotic plaques are associated with increased infiltration of natural killer (NK) cells and higher serum levels of NK activating receptor ligands. Front. Immunol. 10, 1503 (2019).
Nour-Eldine, W. et al. Genetic depletion or hyperresponsiveness of natural killer cells do not affect atherosclerosis development. Circ. Res. 122, 47–57 (2018).
Li, Y. et al. Natural killer cells: friend or foe in metabolic diseases? Front Immunol. 12, 614429 (2021).
Zhao, Y., Zhang, J., Zhang, W. & Xu, Y. A myriad of roles of dendritic cells in atherosclerosis. Clin. Exp. Immunol. 206, 12–27 (2021).
Clement, M. et al. Impaired autophagy in CD11b(+) dendritic cells expands CD4(+) regulatory T cells and limits atherosclerosis in mice. Circ. Res. 125, 1019–1034 (2019).
Sun, Y. et al. Alisol B 23-acetate, a new promoter for cholesterol efflux from dendritic cells, alleviates dyslipidemia and inflammation in advanced atherosclerotic mice. Int. Immunopharmacol. 99, 107956 (2021).
Wang, Y., Song, E., Bai, B. & Vanhoutte, P. M. Toll-like receptors mediating vascular malfunction: lessons from receptor subtypes. Pharm. Ther. 158, 91–100 (2016).
Mann, D. L. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ. Res. 108, 1133–1145 (2011).
Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430, 257–263 (2004).
Koushki, K. et al. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin. Rev. Allergy Immunol. 60, 175–199 (2021).
Lee, C. C., Avalos, A. M. & Ploegh, H. L. Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 12, 168–179 (2012).
Leulier, F. & Lemaitre, B. Toll-like receptors–taking an evolutionary approach. Nat. Rev. Genet. 9, 165–178 (2008).
Lee, J. Y. & Hwang, D. H. The modulation of inflammatory gene expression by lipids: mediation through toll-like receptors. Mol. Cells 21, 174–185 (2006).
Lee, J. Y., Zhao, L. & Hwang, D. H. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr. Rev. 68, 38–61 (2010).
Peiser, L., Mukhopadhyay, S. & Gordon, S. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 14, 123–128 (2002).
Miller, Y. I. et al. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler. Thromb. Vasc. Biol. 25, 1213–1219 (2005).
Bhaskar, S., Sudhakaran, P. R. & Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-kappaB signaling pathway. Cell Immunol. 310, 131–140 (2016).
Curtiss, L. K., Black, A. S., Bonnet, D. J. & Tobias, P. S. Atherosclerosis induced by endogenous and exogenous toll-like receptor (TLR)1 or TLR6 agonists. J. Lipid. Res. 53, 2126–2132 (2012).
Roshan, M. H., Tambo, A. & Pace, N. P. The role of TLR2, TLR4, and TLR9 in the pathogenesis of atherosclerosis. Int J. Inflam. 2016, 1532832 (2016).
Kim, J. et al. The flagellin-TLR5-Nox4 axis promotes the migration of smooth muscle cells in atherosclerosis. Exp. Mol. Med. 51, 1–13 (2019).
Kapelouzou, A. et al. Overexpression of toll-like receptors 2, 3, 4, and 8 is correlated to the vascular atherosclerotic process in the hyperlipidemic rabbit model: the effect of statin treatment. J. Vasc. Res. 54, 156–169 (2017).
Fukuda, D. et al. Toll-like receptor 9 plays a pivotal role in angiotensin II-induced therosclerosis. J. Am. Heart Assoc. 8, e010860 (2019).
Li, B., Xia, Y. & Hu, B. Infection and atherosclerosis: TLR-dependent pathways. Cell Mol. Life Sci. 77, 2751–2769 (2020).
Michelsen, K. S. et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl. Acad. Sci. USA 101, 10679–10684 (2004).
Ding, Y. et al. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32, 1596–1604 (2012).
Bayer, A. L. & Alcaide, P. MyD88: at the heart of inflammatory signaling and cardiovascular disease. J. Mol. Cell Cardiol. 161, 75–85 (2021).
Geng, S. et al. Resolving monocytes generated through TRAM deletion attenuate atherosclerosis. JCI Insight. 6, e149651 (2021).
Huang, B., Park, D. W. & Baek, S. H. TRIF is a regulator of TLR2-induced foam cell formation. Mol. Med. Rep. 14, 3329–3335 (2016).
Yin, Q. Y. et al. Research progress of mechanisms and drug therapy for atherosclerosis on toll-like receptor pathway. J. Cardiovasc. Pharm. 74, 379–388 (2019).
Lehr, H. A. et al. Immunopathogenesis of atherosclerosis: endotoxin accelerates atherosclerosis in rabbits on hypercholesterolemic diet. Circulation 104, 914–920 (2001).
Bahrami, A. et al. Effect of statins on toll-like receptors: a new insight to pleiotropic effects. Pharm. Res. 135, 230–238 (2018).
Lu, Z. et al. TLR4 antagonist reduces early-stage atherosclerosis in diabetic apolipoprotein E-deficient mice. J. Endocrinol. 216, 61–71 (2013).
Monaco, C. et al. Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation 120, 2462–2469 (2009).
Miyamoto, T. et al. Pathogen-accelerated atherosclerosis occurs early after exposure and can be prevented via immunization. Infect. Immun. 74, 1376–1380 (2006).
Binder, C. J. et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 9, 736–743 (2003).
Pothineni, N. V. K. et al. Infections, atherosclerosis, and coronary heart disease. Eur. Heart J. 38, 3195–3201 (2017).
Shah, P. K., Chyu, K. Y., Dimayuga, P. C. & Nilsson, J. Vaccine for atherosclerosis. J. Am. Coll. Cardiol. 64, 2779–2791 (2014).
Sharma, B. R. & Kanneganti, T. D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 22, 550–559 (2021).
Liaqat, A., Asad, M., Shoukat, F. & Khan, A. U. A spotlight on the underlying activation mechanisms of the NLRP3 inflammasome and its role in atherosclerosis: a review. Inflammation 43, 2011–2020 (2020).
Jin, Y. & Fu, J. Novel insights into the NLRP 3 inflammasome in atherosclerosis. J. Am. Heart Assoc. 8, e012219 (2019).
M. Takahashi. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc. Res. 118, 372–385 (2021).
F. Burger, et al. NLRP3 inflammasome activation controls vascular smooth muscle cells phenotypic switch in atherosclerosis. Int. J. Mol. Sci. 23, 340 (2021).
Duncan, J. A. et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. USA 104, 8041–8046 (2007).
Vajjhala, P. R., Mirams, R. E. & Hill, J. M. Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J. Biol. Chem. 287, 41732–41743 (2012).
Yu, H. B. & Finlay, B. B. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe 4, 198–208 (2008).
Christgen, S. & Kanneganti, T. D. Inflammasomes and the fine line between defense and disease. Curr. Opin. Immunol. 62, 39–44 (2020).
Christgen, S., Place, D. E. & Kanneganti, T. D. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 30, 315–327 (2020).
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Di Virgilio, F. et al. The P2X7 receptor in infection and inflammation. Immunity 47, 15–31 (2017).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Yi, Y. S. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 152, 207–217 (2017).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Zeng, C., Wang, R. & Tan, H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int. J. Biol. Sci. 15, 1345–1357 (2019).
Yang, D. et al. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43, 923–932 (2015).
Seok, J. K. et al. Regulation of the NLRP3 inflammasome by post-translational modifications and small molecules. Front. Immunol. 11, 618231 (2020).
Gaidt, M. M. et al. Human monocytes engage an alternative inflammasome pathway. Immunity 44, 833–846 (2016).
Karki, R. et al. IRF8 regulates gram-negative bacteria-mediated NLRP3 inflammasome activation and cell death. J. Immunol. 204, 2514–2522 (2020).
Gurung, P. et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 287, 34474–34483 (2012).
Gurung, P. et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 192, 1835–1846 (2014).
Samir, P. et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature 573, 590–594 (2019).
Vande, L. Walle, et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512, 69–73 (2014).
Malireddi, R. K. S. et al. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J. Exp. Med 215, 1023–1034 (2018).
Briard, B. et al. Galactosaminogalactan activates the inflammasome to provide host protection. Nature 588, 688–692 (2020).
Sharif, H. et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343 (2019).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Song, H. et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat. Commun. 7, 13727 (2016).
Barry, R. et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat. Commun. 9, 3001 (2018).
Shao, L. et al. SUMO1 SUMOylates and SENP3 deSUMOylates NLRP3 to orchestrate the inflammasome activation. FASEB J. 34, 1497–1515 (2020).
Zheng, D., Liwinski, T. & Elinav, E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 6, 36 (2020).
Martinez, G. J., Celermajer, D. S. & Patel, S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis 269, 262–271 (2018).
Yajima, N. et al. Critical role of bone marrow apoptosis-associated speck-like protein, an inflammasome adaptor molecule, in neointimal formation after vascular injury in mice. Circulation 117, 3079–3087 (2008).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Westerterp, M. et al. Cholesterol efflux pathways suppress inflammasome activation, NETosis, and atherogenesis. Circulation 138, 898–912 (2018).
van der Heijden, T. et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1457–1461 (2017).
Menu, P. et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2, e137 (2011).
Chen, S. et al. Sex-specific effects of the Nlrp3 inflammasome on atherogenesis in LDL receptor-deficient mice. JACC Basic Transl. Sci. 5, 582–598 (2020).
Hoseini, Z. et al. NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J. Cell Physiol. 233, 2116–2132 (2018).
Tong, Y. et al. NLRP3 inflammasome and its central role in the cardiovascular diseases. Oxid. Med. Cell Longev. 2020, 4293206 (2020).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Sharma, A. et al. Specific NLRP3 inhibition protects against diabetes-associated atherosclerosis. Diabetes 70, 772–787 (2021).
Zeng, W. et al. The selective NLRP3 inhibitor MCC950 hinders atherosclerosis development by attenuating inflammation and pyroptosis in macrophages. Sci. Rep. 11, 19305 (2021).
Jiang, H. et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214, 3219–3238 (2017).
Li, Y. et al. VX-765 attenuates atherosclerosis in ApoE deficient mice by modulating VSMCs pyroptosis. Exp. Cell Res. 389, 111847 (2020).
C. Stigliano, et al. Methotraxate-loaded hybrid nanoconstructs target vascular lesions and inhibit atherosclerosis progression in ApoE(-/-) mice. Adv Healthc Mater 6, (2017).
Li, C. et al. Site-specific microRNA-33 antagonism by pH-responsive nanotherapies for treatment of atherosclerosis via regulating cholesterol efflux and adaptive immunity. Adv. Funct. Mater. 30, 2002131 (2020).
Zhong, Y. et al. “Plug and Play” functionalized erythrocyte nanoplatform for target atherosclerosis management. ACS Appl. Mater. Interfaces 13, 33862–33873 (2021).
Wang, Y. et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics 11, 164–180 (2021).
Momtazi-Borojeni, A. A. et al. PCSK9 and inflammation: a review of experimental and clinical evidence. Eur. Heart J. Cardiovasc. Pharmacother. 5, 237–245 (2019).
Seidah, N. G., Awan, Z., Chretien, M. & Mbikay, M. PCSK9: a key modulator of cardiovascular health. Circ. Res. 114, 1022–1036 (2014).
Duan, Y. et al. Peroxisome proliferator-activated receptor gamma activation by ligands and dephosphorylation induces proprotein convertase subtilisin kexin type 9 and low density lipoprotein receptor expression. J. Biol. Chem. 287, 23667–23677 (2012).
Seidah, N. G. & Prat, A. The proprotein convertases are potential targets in the treatment of dyslipidemia. J. Mol. Med. 85, 685–696 (2007).
Tao, R. et al. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J. Biol. Chem. 288, 29252–29259 (2013).
Seidah, N. G. The proprotein convertases, 20 years later. Methods Mol. Biol. 768, 23–57 (2011).
Benjannet, S. et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 279, 48865–48875 (2004).
Ragusa, R. et al. PCSK9 and atherosclerosis: looking beyond LDL regulation. Eur. J. Clin. Invest. 51, e13459 (2021).
Ding, Z. et al. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc. Res. 107, 556–567 (2015).
Giunzioni, I. et al. Local effects of human PCSK9 on the atherosclerotic lesion. J. Pathol. 238, 52–62 (2016).
Ding, Z. et al. PCSK9 and inflammation: role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc. Res. 116, 908–915 (2020).
Barale, C., Melchionda, E., Morotti, A. & Russo, I. PCSK9 biology and its role in atherothrombosis. Int J. Mol. Sci. 22, 5880 (2021).
Ding, Z. et al. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid. Redox. Signal. 22, 760–771 (2015).
Ferri, N. et al. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 220, 381–386 (2012).
Ferri, N. et al. PCSK9 knock-out mice are protected from neointimal formation in response to perivascular carotid collar placement. Atherosclerosis 253, 214–224 (2016).
Silverstein, R. L. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Goes “DAMP”. Circulation 143, 62–64 (2021).
Qi, Z. et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) enhances platelet activation, thrombosis, and myocardial infarct expansion by binding to platelet CD36. Circulation 143, 45–61 (2021).
Badimon, L. et al. PCSK9 and LRP5 in macrophage lipid internalization and inflammation. Cardiovasc. Res. 117, 2054–2068 (2021).
Scalise, V. et al. PCSK9 induces tissue factor expression by activation of TLR4/NFkB signaling. Int. J. Mol. Sci. 22, 12640 (2021).
Walley, K. R. et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci. Transl. Med. 6, 258ra143 (2014).
Seidah, N. G. et al. Novel strategies to target proprotein convertase subtilisin kexin 9: beyond monoclonal antibodies. Cardiovasc. Res. 115, 510–518 (2019).
Tombling, B. J. et al. The emerging landscape of peptide-based inhibitors of PCSK9. Atherosclerosis 330, 52–60 (2021).
Nambi, V. & Agha, A. Inclisiran: a game changer in a changing game? J. Am. Coll. Cardiol. 77, 1194–1196 (2021).
Landlinger, C. et al. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice. Eur. Heart J. 38, 2499–2507 (2017).
Kuhnast, S. et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J. Lipid. Res. 55, 2103–2112 (2014).
Wu, D. et al. PCSK9Qbeta-003 vaccine attenuates atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Drugs Ther. 35, 141–151 (2021).
Sahebkar, A., Momtazi-Borojeni, A. A. & Banach, M. PCSK9 vaccine: so near, yet so far! Eur. Heart J. 42, 4007–4010 (2021).
Momtazi-Borojeni, A. A., Jaafari, M. R., Badiee, A. & Sahebkar, A. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis 283, 69–78 (2019).
Momtazi-Borojeni, A. A., Jaafari, M. R., Badiee, A., Banach, M. & Sahebkar, A. Therapeutic effect of nanoliposomal PCSK9 vaccine in a mouse model of atherosclerosis. BMC Med. 17, 223 (2019).
Momtazi-Borojeni, A. A., Jaafari, M. R., Afshar, M., Banach, M. & Sahebkar, A. PCSK9 immunization using nanoliposomes: preventive efficacy against hypercholesterolemia and atherosclerosis. Arch. Med. Sci. 17, 1365–1377 (2021).
Bray, S. J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 (2006).
Six, E. et al. The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc. Natl. Acad. Sci. USA 100, 7638–7643 (2003).
Ayaz, F. & Osborne, B. A. Non-canonical notch signaling in cancer and immunity. Front. Oncol. 4, 345 (2014).
Vieceli Dalla Sega, F. et al. Notch signaling regulates immune responses in atherosclerosis. Front Immunol. 10, 1130 (2019).
Lee, K. S. et al. Roles of PINK1, mTORC2, and mitochondria in preserving brain tumor-forming stem cells in a noncanonical Notch signaling pathway. Genes Dev. 27, 2642–2647 (2013).
Quillard, T. & Charreau, B. Impact of notch signaling on inflammatory responses in cardiovascular disorders. Int. J. Mol. Sci. 14, 6863–6888 (2013).
Fior, R. & Henrique, D. “Notch-Off”: a perspective on the termination of Notch signalling. Int. J. Dev. Biol. 53, 1379–1384 (2009).
Nus, M. et al. Endothelial Jag1-RBPJ signalling promotes inflammatory leucocyte recruitment and atherosclerosis. Cardiovasc. Res. 112, 568–580 (2016).
Mack, J. J. & Iruela-Arispe, M. L. NOTCH regulation of the endothelial cell phenotype. Curr. Opin. Hematol. 25, 212–218 (2018).
Mack, J. J. et al. NOTCH1 is a mechanosensor in adult arteries. Nat. Commun. 8, 1620 (2017).
Fortini, F. et al. Estrogen receptor beta-dependent Notch1 activation protects vascular endothelium against tumor necrosis factor alpha (TNFalpha)-induced apoptosis. J. Biol. Chem. 292, 18178–18191 (2017).
Fortini, F. et al. Estrogen-mediated protection against coronary heart disease: the role of the Notch pathway. J. Steroid. Biochem. Mol. Biol. 189, 87–100 (2019).
Polacheck, W. J. et al. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 552, 258–262 (2017).
Fortini, F. et al. Well-known and novel players in endothelial dysfunction: updates on a Notch(ed) landscape. Biomedicines 9, 997 (2021).
Boucher, J., Gridley, T. & Liaw, L. Molecular pathways of notch signaling in vascular smooth muscle cells. Front. Physiol. 3, 81 (2012).
Mao, C. et al. Nidogen-2 maintains the contractile phenotype of vascular smooth muscle cells and prevents neointima formation via bridging jagged1-Notch3 signaling. Circulation 144, 1244–1261 (2021).
Martos-Rodriguez, C. J. et al. Fibrous caps in atherosclerosis form by notch-dependent mechanisms common to arterial media development. Arterioscler. Thromb. Vasc. Biol. 41, e427–e439 (2021).
Hedin, U. Another Notch in the cap. Arterioscler. Thromb. Vasc. Biol. 41, 2384–2386 (2021).
Singla, R. D., Wang, J. & Singla, D. K. Regulation of Notch 1 signaling in THP-1 cells enhances M2 macrophage differentiation. Am. J. Physiol. Heart Circ. Physiol. 307, H1634–H1642 (2014).
Singla, D. K., Wang, J. & Singla, R. Primary human monocytes differentiate into M2 macrophages and involve Notch-1 pathway. Can. J. Physiol. Pharm. 95, 288–294 (2017).
Aoyama, T. et al. gamma-Secretase inhibitor reduces diet-induced atherosclerosis in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 383, 216–221 (2009).
Hao, Y. et al. Inhibition of notch enhances the anti-atherosclerotic effects of LXR agonists while reducing fatty liver development in ApoE-deficient mice. Toxicol. Appl. Pharm. 406, 115211 (2020).
Wongchana, W. & Palaga, T. Direct regulation of interleukin-6 expression by Notch signaling in macrophages. Cell Mol. Immunol. 9, 155–162 (2012).
Hildebrand, D. et al. The interplay of notch signaling and STAT3 in TLR-activated human primary monocytes. Front. Cell Infect. Microbiol 8, 241 (2018).
Christopoulos, P. F. et al. Targeting the Notch signaling pathway in chronic inflammatory diseases. Front. Immunol. 12, 668207 (2021).
Carter, P. J. & Lazar, G. A. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 17, 197–223 (2018).
Tran, I. T. et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J. Clin. Invest. 123, 1590–1604 (2013).
Wu, Y. et al. Therapeutic antibody targeting of individual Notch receptors. Nature 464, 1052–1057 (2010).
Fukuda, D. et al. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc. Natl. Acad. Sci. USA 109, E1868–E1877 (2012).
Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Schulte, G. & Bryja, V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharm. Sci. 28, 518–525 (2007).
Janda, C. Y. et al. Structural basis of Wnt recognition by Frizzled. Science 337, 59–64 (2012).
Lorzadeh, S., Kohan, L., Ghavami, S. & Azarpira, N. Autophagy and the Wnt signaling pathway: a focus on Wnt/beta-catenin signaling. Biochim. Biophys. Acta Mol. Cell Res 1868, 118926 (2021).
Albanese, I. et al. Atherosclerotic calcification: Wnt is the hint. J. Am. Heart Assoc. 7, e007356 (2018).
Boucher, P., Matz, R. L. & Terrand, J. atherosclerosis: gone with the Wnt? Atherosclerosis 301, 15–22 (2020).
Mani, A. et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315, 1278–1282 (2007).
Singh, R. et al. Rare nonconservative LRP6 mutations are associated with metabolic syndrome. Hum. Mutat. 34, 1221–1225 (2013).
Goliasch, G. et al. Premature myocardial infarction is associated with low serum levels of Wnt-1. Atherosclerosis 222, 251–256 (2012).
Weinstock, A. et al. Wnt signaling enhances macrophage responses to IL-4 and promotes resolution of atherosclerosis. Elife 10, e67932 (2021).
Zhang, Y. et al. Dickkopf-2 knockdown protects against classic macrophage polarization and lipid loading by activation of Wnt/beta-catenin signaling. J. Cardiol. 78, 328–333 (2021).
Gan, Y. R. et al. Dickkopf1/cysteinerich angiogenic inducer 61 axis mediates palmitic acid induced inflammation and apoptosis of vascular endothelial cells. Mol. Med. Rep. 23, 122 (2021).
Li, A. et al. Low shear stress-induced endothelial mesenchymal transformation via the down-regulation of TET2. Biochem. Biophys. Res. Commun. 545, 20–26 (2021).
Weerackoon, N., Gunawardhana, K. L. & Mani, A. Wnt signaling cascades and their role in coronary artery health and disease. J. Cell Signal. 2, 52–62 (2021).
Sun, H. et al. Down-regulation of microRNA-342-5p or up-regulation of wnt3a inhibits angiogenesis and maintains atherosclerotic plaque stability in atherosclerosis mice. Nanoscale Res. Lett. 16, 165 (2021).
Borrell-Pages, M., Romero, J. C., Juan-Babot, O. & Badimon, L. Wnt pathway activation, cell migration, and lipid uptake is regulated by low-density lipoprotein receptor-related protein 5 in human macrophages. Eur. Heart J. 32, 2841–2850 (2011).
Singla, B. et al. Role of R-spondin 2 in arterial lymphangiogenesis and atherosclerosis. Cardiovasc. Res. 117, 1489–1509 (2021).
Siman-Tov, R. et al. Circulating Wnt ligands activate the wnt signaling pathway in mature erythrocytes. Arterioscler. Thromb. Vasc. Biol. 41, e243–e264 (2021).
Terenzi, D. C., Verma, S. & Hess, D. A. Exploring the clinical implications of Wnt signaling in enucleated erythrocytes. Arterioscler. Thromb. Vasc. Biol. 41, 1654–1656 (2021).
Mach, F. et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 41, 111–188 (2020).
Schwartz, G. G. et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 379, 2097–2107 (2018).
Raal, F. J., Hovingh, G. K. & Catapano, A. L. Familial hypercholesterolemia treatments: Guidelines and new therapies. Atherosclerosis 277, 483–492 (2018).
Kolovou, V. et al. Lipoprotein apheresis and proprotein convertase subtilisin/Kexin Type 9 inhibitors in patients with heterozygous familial hypercholesterolemia: a one center study. J. Cardiovasc. Pharm. Ther. 26, 51–58 (2021).
AlTurki, A. et al. Meta-analysis of randomized controlled trials assessing the impact of proprotein convertase subtilisin/kexin type 9 antibodies on mortality and cardiovascular outcomes. Am. J. Cardiol. 124, 1869–1875 (2019).
Casula, M. et al. Cardiovascular events with PCSK9 inhibitors: an updated meta-analysis of randomised controlled trials. Pharm. Res 143, 143–150 (2019).
Rifai, M. A. & Ballantyne, C. M. PCSK9-targeted therapies: present and future approaches. Nat. Rev. Cardiol. 18, 805–806 (2021).
Ray, K. K. et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N. Engl. J. Med 382, 1507–1519 (2020).
Ummarino, D. Dyslipidaemia: Anti-PCSK9 vaccines to halt atherosclerosis. Nat. Rev. Cardiol. 14, 442–443 (2017).
Zeitlinger, M. et al. A phase I study assessing the safety, tolerability, immunogenicity, and low-density lipoprotein cholesterol-lowering activity of immunotherapeutics targeting PCSK9. Eur. J. Clin. Pharm. 77, 1473–1484 (2021).
Kersten, S. ANGPTL3 as therapeutic target. Curr. Opin. Lipido. 32, 335–341 (2021).
Gaudet, D. et al. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N. Engl. J. Med. 377, 296–297 (2017).
Graham, M. J. et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N. Engl. J. Med. 377, 222–232 (2017).
Wilkins, J. T. & Lloyd-Jones, D. M. Novel lipid-lowering therapies to reduce cardiovascular risk. JAMA 326, 266–267 (2021).
Agarwala, A. & Goldberg, A. C. Bempedoic acid: a promising novel agent for LDL-C lowering. Future Cardiol. 16, 361–371 (2020).
Ballantyne, C. M. et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis 277, 195–203 (2018).
Goldberg, A. C. et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom Randomized Clinical trial. JAMA 322, 1780–1788 (2019).
Perera, K. et al. Bempedoic acid for high-risk patients with CVD as adjunct lipid-lowering therapy: a cost-effectiveness analysis. J. Clin. Lipido. 14, 772–783 (2020).
Gupta, M. et al. Novel emerging therapies in atherosclerosis targeting lipid metabolism. Expert Opin. Investig. Drugs 29, 611–622 (2020).
Berberich, A. J. & Hegele, R. A. Lomitapide for the treatment of hypercholesterolemia. Expert Opin. Pharmacother. 18, 1261–1268 (2017).
Yahya, R. et al. Lomitapide affects HDL composition and function. Atherosclerosis 251, 15–18 (2016).
Harada-Shiba, M. et al. Efficacy and safety of Lomitapide in Japanese patients with homozygous familial hypercholesterolemia. J. Atheroscler. Thromb. 24, 402–411 (2017).
Meyers, C. D. et al. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis. 14, 8 (2015).
Meyers, C. D., Amer, A., Majumdar, T. & Chen, J. Pharmacokinetics, pharmacodynamics, safety, and tolerability of pradigastat, a novel diacylglycerol acyltransferase 1 inhibitor in overweight or obese, but otherwise healthy human subjects. J. Clin. Pharm. 55, 1031–1041 (2015).
Moriarty, P. M., Gray, J. V. & Gorby, L. K. Lipoprotein apheresis for lipoprotein(a) and cardiovascular disease. J. Clin. Lipido. 13, 894–900 (2019).
Merki, E. et al. Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipoprotein (a) in transgenic mice. J. Am. Coll. Cardiol. 57, 1611–1621 (2011).
Fogacci, F. et al. Efficacy and safety of mipomersen: a systematic review and meta-analysis of randomized clinical trials. Drugs 79, 751–766 (2019).
Reeskamp, L. F. et al. Safety and efficacy of mipomersen in patients with heterozygous familial hypercholesterolemia. Atherosclerosis 280, 109–117 (2019).
Astaneh, B., Makhdami, N., Astaneh, V. & Guyatt, G. The effect of mipomersen in the management of patients with familial hypercholesterolemia: a systematic review and meta-analysis of clinical trials. J. Cardiovasc. Dev. Dis. 8, 82 (2021).
Aslesh, T. & Yokota, T. Development of antisense oligonucleotide gapmers for the treatment of dyslipidemia and lipodystrophy. Methods Mol. Biol. 2176, 69–85 (2020).
Yang, X. et al. Reduction in lipoprotein-associated apoC-III levels following volanesorsen therapy: phase 2 randomized trial results. J. Lipid Res. 57, 706–713 (2016).
Darabi, M., Guillas-Baudouin, I., Le Goff, W., Chapman, M. J. & Kontush, A. Therapeutic applications of reconstituted HDL: When structure meets function. Pharm. Ther. 157, 28–42 (2016).
Easton, R. et al. A multiple ascending dose study of CSL112, an infused formulation of ApoA-I. J. Clin. Pharm. 54, 301–310 (2014).
Tricoci, P. et al. Infusion of reconstituted high-density lipoprotein, CSL112, in patients with atherosclerosis: safety and pharmacokinetic results from a phase 2a randomized clinical trial. J. Am. Heart Assoc. 4, e002171 (2015).
Michael Gibson, C. et al. Safety and tolerability of CSL112, a reconstituted, infusible, plasma-derived apolipoprotein a-i, after acute myocardial infarction: the AEGIS-I trial (ApoA-I event reducing in ischemic syndromes I). Circulation 134, 1918–1930 (2016).
Shamburek, R. D. et al. Safety and tolerability of ACP-501, a recombinant human lecithin:cholesterol acyltransferase, in a phase 1 single-dose escalation study. Circ. Res 118, 73–82 (2016).
Shamburek, R. D. et al. Familial lecithin:cholesterol acyltransferase deficiency: first-in-human treatment with enzyme replacement. J. Clin. Lipido. 10, 356–367 (2016).
M. P. Bonaca, et al. Recombinant human Lecithin-Cholesterol acyltransferase in patients with atherosclerosis: phase 2a primary results and phase 2b design. Eur. Heart J. Cardiovasc. Pharmacother. (2021). [Online ahead of print]
Ishibashi, S. et al. Effects of K-877, a novel selective PPARalpha modulator (SPPARMalpha), in dyslipidaemic patients: a randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis 249, 36–43 (2016).
Yamashita, S., Masuda, D. & Matsuzawa, Y. Pemafibrate, a new selective PPARalpha modulator: drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr. Atheroscler. Rep. 22, 5 (2020).
Gaudet, D., Drouin-Chartier, J. P. & Couture, P. Lipid metabolism and emerging targets for lipid-lowering therapy. Can. J. Cardiol. 33, 872–882 (2017).
Stein, E., Bays, H., Koren, M., Bakker-Arkema, R. & Bisgaier, C. Efficacy and safety of gemcabene as add-on to stable statin therapy in hypercholesterolemic patients. J. Clin. Lipido. 10, 1212–1222 (2016).
Larsen, L. E., Stoekenbroek, R. M., Kastelein, J. J. P. & Holleboom, A. G. Moving targets: recent advances in lipid-lowering therapies. Arterioscler. Thromb. Vasc. Biol. 39, 349–359 (2019).
Valanti, E. K. et al. Advances in biological therapies for dyslipidemias and atherosclerosis. Metabolism 116, 154461 (2021).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2019).
Drucker, D. J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27, 740–756 (2018).
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Cowart, K., Gonzalez, R. & Carris, N. W. Cardiovascular and microvascular outcomes with iGlarLixi versus iDegLira: a real-world, population-based cohort study. Diabetes Obes. Metab. 24, 348–353 (2022).
Knudsen, L. B. & Lau, J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol. (Lausanne) 10, 155 (2019).
Rosen, C. J. & Ingelfinger, J. R. Once-weekly semaglutide in adults with overweight or obesity. Reply. N. Engl. J. Med. 385, e4 (2021).
Ferrari, F., Scheffel, R. S., Martins, V. M., Santos, R. D. & Stein, R. Glucagon-like peptide-1 receptor agonists in type 2 diabetes mellitus and cardiovascular disease: the past, present, and future. Am. J. Cardiovasc. Drugs (2021). [Online ahead of print].
DeFronzo, R. A. et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36, 3169–3176 (2013).
Toyama, T. et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes. Metab. 21, 1237–1250 (2019).
Pereira, M. J. & Eriksson, J. W. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs 79, 219–230 (2019).
Cannon, C. P. et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N. Engl. J. Med. 383, 1425–1435 (2020).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 (2019).
Frampton Empagliflozin, J. E. A review in type 2 diabetes. Drugs 78, 1037–1048 (2018).
Neal, B. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017).
Wang, H. et al. DPP-4 inhibitor linagliptin ameliorates oxidized LDL-induced THP-1 macrophage foam cell formation and inflammation. Drug Des. Dev. Ther. 14, 3929–3940 (2020).
Duan, L. et al. The regulatory role of DPP4 in atherosclerotic disease. Cardiovasc. Diabetol. 16, 76 (2017).
Abd El Aziz, M. S., Kahle, M., Meier, J. J. & Nauck, M. A. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes. Metab. 19, 216–227 (2017).
Nauck, M. A. et al. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 136, 849–870 (2017).
Subrahmanyan, N. A., Koshy, R. M., Jacob, K. & Pappachan, J. M. Efficacy and cardiovascular safety of DPP-4 inhibitors. Curr. Drug Safety. 16, 154–164 (2021).
Koibuchi, N. et al. DPP-4 inhibitor linagliptin ameliorates cardiovascular injury in salt-sensitive hypertensive rats independently of blood glucose and blood pressure. Cardiovasc. Diabetol. 13, 157 (2014).
Aroor, A. R., Manrique-Acevedo, C. & DeMarco, V. G. The role of dipeptidylpeptidase-4 inhibitors in management of cardiovascular disease in diabetes; focus on linagliptin. Cardiovasc. Diabetol. 17, 59 (2018).
Schurmann, C. et al. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J. Pharm. Exp. Ther. 342, 71–80 (2012).
Rosenstock, J. et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA 322, 1155–1166 (2019).
Rosenstock, J. et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 321, 69–79 (2019).
Green, J. B. et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 373, 232–242 (2015).
Deacon, C. F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes. Metab. 13, 7–18 (2011).
Rehman, M. B. et al. Efficacy and safety of DPP-4 inhibitors in patients with type 2 diabetes: meta-analysis of placebo-controlled randomized clinical trials. Diabetes Metab. 43, 48–58 (2017).
Toh, S. et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann. Intern. Med 164, 705–714 (2016).
Arnetz, L. et al. Copeptin, insulin-like growth factor binding protein-1 and sitagliptin: a report from the BEta-cell function in glucose abnormalities and acute myocardial infarction study. Diab Vasc. Dis. Res. 13, 307–311 (2016).
Yang, T. Y. et al. Association of Sitagliptin with cardiovascular outcome in diabetic patients: a nationwide cohort study. Acta Diabetol. 53, 461–468 (2016).
Bhaskar, V. et al. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis 216, 313–320 (2011).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Hoffman, H. M. & Broderick, L. The role of the inflammasome in patients with autoinflammatory diseases. J. Allergy Clin. Immunol. 138, 3–14 (2016).
Pergola, P. E. et al. Ziltivekimab for treatment of anemia of inflammation in patients on hemodialysis: results from a phase 1/2 multicenter, randomized, double-blind, placebo-controlled trial. J. Am. Soc. Nephrol. 32, 211–222 (2021).
Rubbert-Roth, A., Furst, D. E., Nebesky, J. M., Jin, A. & Berber, E. A review of recent advances using tocilizumab in the treatment of rheumatic diseases. Rheumatol. Ther. 5, 21–42 (2018).
Lee, E. B. A review of sarilumab for the treatment of rheumatoid arthritis. Immunotherapy 10, 57–65 (2018).
Castagne, B. et al. Cardiovascular safety of tocilizumab: a systematic review and network meta-analysis. PLoS One 14, e0220178 (2019).
Yeung, Y. T., Aziz, F., Guerrero-Castilla, A. & Arguelles, S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 24, 1449–1484 (2018).
Micha, R. et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am. J. Cardiol. 108, 1362–1370 (2011).
Xie, F. et al. Benefits of methotrexate use on cardiovascular disease risk among rheumatoid arthritis patients initiating biologic disease-modifying antirheumatic drugs. J. Rheumatol. 48, 804–812 (2021).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Opstal, T. S. J. et al. Colchicine attenuates inflammation beyond the inflammasome in chronic coronary artery disease: a LoDoCo2 proteomic substudy. Circulation 142, 1996–1998 (2020).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Wohlford, G. F. et al. Phase 1B, randomized, double-blinded, dose escalation, single-center, repeat dose safety and pharmacodynamics study of the oral NLRP3 inhibitor dapansutrile in subjects with NYHA II-III systolic heart failure. J. Cardiovasc. Pharm. 77, 49–60 (2020).
Del Buono, M. G., Crea, F., Versaci, F. & Biondi-Zoccai, G. NLRP3 inflammasome: a new promising therapeutic target to treat heart failure. J. Cardiovasc. Pharm. 77, 159–161 (2021).
O’Donoghue, M. L. et al. Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. JAMA 315, 1591–1599 (2016).
O’Donoghue, M. L. et al. Rationale and design of the LosmApimod To Inhibit p38 MAP kinase as a TherapeUtic target and moDify outcomes after an acute coronary syndromE trial. Am. Heart J. 169, 622–630 e6 (2015).
Tun, B. & Frishman, W. H. Effects of anti-inflammatory medications in patients with coronary artery disease: a focus on losmapimod. Cardiol. Rev. 26, 152–156 (2018).
Antonopoulos, A. S. et al. Anti-inflammatory agents in peripheral arterial disease. Curr. Opin. Pharm. 39, 1–8 (2018).
Wassel, C. L. et al. Soluble P-selectin predicts lower extremity peripheral artery disease incidence and change in the ankle brachial index: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 239, 405–411 (2015).
Casella, I. B. & Presti, C. A new era of medical therapy for peripheral artery disease. J. Vasc. Bras. 19, e20190056 (2020).
Fras, Z., Trsan, J. & Banach, M. On the present and future role of Lp-PLA2 in atherosclerosis-related cardiovascular risk prediction and management. Arch. Med Sci. 17, 954–964 (2021).
Santoso, A., Heriansyah, T. & Rohman, M. S. Phospholipase A2 is an inflammatory predictor in cardiovascular diseases: is there any spacious room to prove the causation? Curr. Cardiol. Rev. 16, 3–10 (2020).
Toledo-Ibelles, P. & Mas-Oliva, J. Antioxidants in the fight against atherosclerosis: is this a dead end? Curr. Atheroscler. Rep. 20, 36 (2018).
Tardif, J. C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (91739301, 91849102); the Key Natural Science Foundation Projects of Hebei Province (H2019206028); Natural Science Foundation of Hebei Province (H2021206006); Natural Science Youth Fund of Hebei Province Education Department (QN2020167).
Author information
Authors and Affiliations
Contributions
M.H. conceived and modified the manuscript. P.K., D.D.Z. and R.J.G. prepared the manuscript. X.F.H. and Z.Y.C. prepared the figures. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kong, P., Cui, ZY., Huang, XF. et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Sig Transduct Target Ther 7, 131 (2022). https://doi.org/10.1038/s41392-022-00955-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-022-00955-7
This article is cited by
-
Association of plasma proteomics with incident coronary heart disease in individuals with and without type 2 diabetes: results from the population-based KORA study
Cardiovascular Diabetology (2024)
-
Insulin resistance and coronary inflammation in patients with coronary artery disease: a cross-sectional study
Cardiovascular Diabetology (2024)
-
The effect of curcumin and high-content eicosapentaenoic acid supplementations in type 2 diabetes mellitus patients: a double-blinded randomized clinical trial
Nutrition & Diabetes (2024)
-
Multifunctional nanoparticle-mediated combining therapy for human diseases
Signal Transduction and Targeted Therapy (2024)
-
Cardiovascular disease and cancer: shared risk factors and mechanisms
Nature Reviews Cardiology (2024)