Abstract
Study design
Narrative review.
Objectives
The purpose is to review the organisation of the nerve pathways that control defecation and to relate this knowledge to the deficits in colorectal function after SCI.
Methods
A literature review was conducted to identify salient features of defecation control pathways and the functional consequences of damage to these pathways in SCI.
Results
The control pathways for defecation have separate pontine centres under cortical control that influence defecation. The pontine centres connect, separately, with autonomic preganglionic neurons of the spinal defecation centres and somatic motor neurons of Onuf’s nucleus in the sacral spinal cord. Organised propulsive motor patterns can be generated by stimulation of the spinal defecation centres. Activation of the somatic neurons contracts the external sphincter. The analysis aids in interpreting the consequences of SCI and predicts therapeutic strategies.
Conclusions
Analysis of the bowel control circuits identifies sites at which bowel function may be modulated after SCI. Colokinetic drugs that elicit propulsive contractions of the colorectum may provide valuable augmentation of non-pharmacological bowel management procedures.
Similar content being viewed by others
Introduction
This paper provides a contemporary review of the central and peripheral components of the control pathways for faecal continence and defecation that is currently lacking. In particular we relate these pathways to the changes seen after spinal cord injury and consider sites to which drugs that could be used in the treatment of fecal retention after spinal cord injury could be targeted.
Methods
Literature reviews were conducted using PubMed and Google Scholar. We searched for papers published after 1974. When relevant papers were identified, earlier literature referred to was sought out. Searches combining the terms defecation and/or micturition with spinal cord injury, spinal cord, CNS and pathway tracing were used. We also investigated citing literature that was related to publications that were identified in the searches. Literature has been evaluated for relevance to analysis of neural pathways for defecation control and for relevance to the bowel consequences experienced by people living with spinal cord injury.
Results
Changed bowel function in SCI
The majority of spinal injuries occur early in life, and a high proportion of injuries that lead to para- or tetraplegia compromise bowel function. It has been estimated that disturbances of bowel function occur in over 80% of people with spinal cord injury (SCI) [1]. Challenges related to living with the bowel complications of SCI include costs, emotional impact, diet, education and employment, intimacy and interpersonal relations, social participation, spontaneity and daily schedule, travel, lack of appropriate and consistent assistance, loss of autonomy (independence, privacy), lack of predictability and fear of incontinence, medical complications, pain or discomfort, physical effort of the bowel routine, physical experience, and time requirements to deal with bowel problems [2, 3].
A major problem identified in SCI is an inability to empty the bowel when defecation is convenient and a leakage of bowel contents (overflow incontinence) that occurs at inappropriate times [4,5,6,7]. Over half of all SCI patients with an injury above L2 suffer from constipation [8]. Constipation and fecal leakage is highly inconvenient and often embarrassing for the patient. Surveys indicate that bowel problems are amongst the commonest secondary effects of SCI that trouble people living with SCI [2, 9,10,11,12]. Failure of normal bowel control can have significant downstream clinical effects, including impaction, haemorrhoids, rectal bleeding, prolapse, formation of anal fissures and chronic constipation leading to megacolon requiring operative diversion [5].
The neural pathways for volitional bowel control
Although we emphasise control pathways in humans, we have relied on studies in other species for some aspects of the neural circuits. Many of the detailed studies of the nerve pathways that control colorectal function have been made in rats and other laboratory animals. Rats are tetrapedal. They do not stop normal activity to defecate, nor do they adopt a defecatory posture. Thus, they exhibit a different control over defecatory behaviour when compared to humans, who normally retain faeces and defecate only intermittently. An upright, bipedal posture is also associated with differences in the structure and functional characteristics of the pelvic floor of humans compared with tetrapods, reflecting its important role in continence imposed by an upright posture [13]. Pelvic floor differences include the greater prominence of the puborectalis and the presence of slow twitch fibres in the external anal sphincter of humans. Nevertheless, the organisation of the nerve circuits that control defecation show similarities between species.
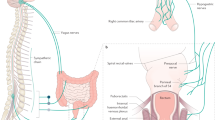
Voluntary (conscious) defecation is controlled from the cerebral cortex, which allows humans to make a decision to defecate or not when the urge is sensed. Two pathways are activated: (i) an autonomic pathway that controls propulsive activity of the smooth muscle of the colorectum and relaxation of the internal anal sphincter that is co-ordinated with propulsive activity and (2) a somatic pathway to the striated muscle of the external anal sphincter that is initially constricted and then relaxed under descending volitional control (Fig. 1). The autonomic pathways impinge on enteric reflex pathways that can cause propulsion even in the absence of central input. These are discussed in the context of involuntary reflexes below. Once the decision to defecate has been made the message travels from the cortex via the spinal cord [14]. In the spinal cord, control of the colorectum is relayed through the sacral spinal defecation centre [15, 16] and control of the external sphincter is relayed through Onuf’s nucleus in the sacral cord [17]. Signals from the spinal defecation centre direct movements of the wall of the gut to cause bowel emptying (Fig. 1). The extrinsic anal sphincter relaxes in co-ordination with colorectal propulsion.
The nerve pathways for voluntary control of defecation and fecal continence. Cortical centres that govern voluntary control provide inputs that either inhibit or enhance excitability of neurons in the brain stem, a medial nucleus (Barrington’s nucleus, BN) through which autonomic pathways to the distal colon and rectum are activated, and a lateral cell group (LCG) that controls the external anal sphincter. The medial group of neurons projects to the spinal defecation centre in the intermediolateral column (IML) at S1 level. This in turn connects with intrinsic reflex pathways of the enteric nervous system (ENS), via the pelvic ganglia. Afferent (sensory) neurons that detect pressure and mucosal irritation in the colon contribute to urge and neurons that sense pressure in the abdominal cavity enhance defecation. These connect to second order (2 ord) neurons that make local connections in the spinal cord and provide sensory information to the pons and cortex. Descending neurons from the LCG synapse in Onuf’s nucleus (ON) on motor neurons that supply the external sphincter
The brain stem centre for control of the smooth muscle of the colorectum and internal anal sphincter is Barrington’s nucleus, which lies near the floor of the fourth ventricle, adjacent to the locus coeruleus [18]. Although most of the information on the roles of this nucleus comes from animal studies, lesion and imaging studies in human identify a region in the human brain stem for the control of micturition that is equivalent to Barrington’s nucleus [13, 19]. While animal studies suggest overlap of micturition with defecatory control, no specific evidence exists for the role of Barrington’s nucleus in human defecation control. In animals, neurons in Barrington’s nucleus are activated by colon distension and by corticotrophin releasing factor, and are subject to over-riding cortical control. Stimulation of Barrington’s nucleus increases colonic intraluminal pressure [20] and injection of radioactive amino acids into Barrington’s nucleus labels nerve fibres surrounding preganglionic neurons in the region of the lumbosacral defecation centre [21]. Barrington’s nucleus contains both the pontine defecation centre and the pontine micturition centre and it has not been possible to distinguish neurons with bladder and bowel controlling functions by morphology, size, position or chemistry in this nucleus. Injection of retrograde label into the defecation centre region labels nerve cells in Barrington’s nucleus, as does the injection of trans-synaptic retrograde tracer into the wall of the colon [15]. Labelling of Barrington’s nucleus from the colon was prevented by spinal cord transection. The projection from the pontine defecation centre to the lumbosacral defecation centre is bilateral [22], but the neurons from the pontine defecation centre do not innervate Onuf’s nucleus, the nucleus through which the external sphincter is controlled [23]. Inputs to the region of the pontine defecation centre come from neurons at the same level (ponto-medullary neurons), the hypothalamus and the cerebral cortex, notably the medial-frontal cortex [23,24,25,26]. Consistent with this neuroanatomy, injection of trans-synaptically transported retrograde tracer into the colon labelled cells in the medial-frontal cortex [15]. Neurons in the caudal nucleus raphe obscurus (nRO), in the midline of the lower brain stem, also project to the defecation centres [25]. When the nRO is electrically stimulated, these pathways inhibit colorectal motility, and thus may be involved in maintaining continence, and also inhibit internal and external anal sphincter contraction, which could be permissive to defecation. Electrical stimulation is rather a blunt tool, and could activate more than one mechanism emanating from nRO. It is notable that injection of tracer into the colon of spinally transected rats labelled neurons of the area postrema, nucleus of the solitary tract, dorsal nucleus of the vagus and nucleus ambiguus, but not Barrington’s nucleus [15]. This indicates that there is a vagal innervation of the colon. However, the vagal pathways have little or no role in voluntary defecation, which is lost after significant damage to the spinal cord.
Onuf’s nucleus, which supplies the muscles of the pelvic floor that form the external anal and external urethral sphincters, is also innervated by motor pathways from the ponto-medullary region [18, 27]. Within Onuf’s nucleus, regions containing motor neurons to the external anal and external urethral sphincters are distinct [28,29,30]. In the cat, the ponto-medullary premotor neurons supplying Onuf’s nucleus are laterally located, and are distinct from the medially located neurons of Barrington’s nucleus, with which they make no direct connection [17], whereas in the rat the cell groups are close or intermingled [27]. It is concluded from the cat data that co-ordination of colon and extrinsic sphincter movements occurs at centres that in turn connect with these two neuron groups [17]. In uninjured individuals, cortical inhibition of external anal sphincter contractile tone occurs in a co-ordinated fashion on straining [6]. There is evidence for studies of human volunteers that connections in the spinal cord, independently of descending pathways from the brain, can influence the external sphincter [31]. The authors propose that afferents in the rectum initiate contractions of the external sphincter through a spinal reflex. Consistent with the presence of local reflex control of the external sphincter, there are connections to Onuf’s nucleus from spinal afferents and the region of the defecation centre at the sacral level [32]. Some of the local inputs to Onuf’s nucleus are inhibitory.
Defecation is facilitated by increasing intra-abdominal pressure or by irritation of the mucosal lining of the rectum [33], indicating that sensory pathways from the abdomen and rectum impinge on the defecation control pathways. This facilitation can assist defecation in spinal injured patients, but not if the injury is below the conus, i.e., below the spinal defecation centres [34]. This agrees with the anatomy, in which the afferents project to the lumbosacral spinal cord (Fig. 1).
Therefore, the act of defecation is co-ordinated by interaction between the brain, spinal cord, enteric neurons, and the muscle of the colon, rectum, anus, and pelvic floor and is dependent on conscious control.
Bowel control after spinal cord injury
Because the pathways for voluntary control of the colorectum and external sphincter run down the spinal cord, voluntary control of fecal continence and defecation is disrupted by SCI, to varying extents dependent on the completeness of the interruption. However, involuntary portions of the defecation reflex remain intact after SCI, notably after supraconal SCI. The spinal defecation centre, located in the sacral cord, is usually intact following SCI, as approximately 90% of traumatic spinal cord injuries are at cervical or thoracic levels [35]. Moreover, the enteric nervous system (ENS) and its reflex pathways remain intact (Fig. 1). Comparison of colonic myoelectric activity between a group of para- or tetraplegic subjects, ranging from 3 months to 11 years post injury, and uninjured individuals showed that there is no loss of colonic muscle contractility [36], consistent with the muscle retaining its enteric innervation. In fact, the basal myoelectrical activity was slightly, about 25%, higher in the injured subjects compared to controls. This may be contributed to by a loss of the central inhibition that contributes to fecal continence.
The recto-anal reflex, which relies on ENS nerve circuits, causes the internal anal sphincter to relax when the colon is distended or the colonic mucosa is stimulated mechanically or chemically [33, 37]. Anorectal manometry demonstrates normal internal anal sphincter relaxation after rectal distention in people with SCI, demonstrating intact enteric defecation reflexes [36]. If the injury is distal to the S1 defecation centres (infraconal), the colorectal reflexes are deficient [5]. This suggests that in intact humans, the co-ordinated propulsive activity of the colorectum depends on command signals from the sacral defecation centre [5]. Individuals with injury below the defecation centre present with slow stool propulsion, hypotonic anal sphincter, deficient recto-colic reflex, constipation and incontinence [5]. Consistent with these results, studies in cats show that activation of colorectal afferents causes propulsive contractile activity in the colon that depends on the pelvic nerve being intact and which persists after transection of the cord rostral to the defection centres [38].
A study of 23 subjects with complete traumatic spinal injury (at C6-L1) with age matched controls reported that external anal sphincter response to rectal distension was noticeably attenuated [39]. This suggests that adequate control of the external sphincter is dependent on descending spinal pathways, as the organisation of the nerve circuits suggests (Fig. 1). However, the changes in external sphincter functions are quite variable between patients, and can be influenced by trans-synaptic changes that depend on time since injury [40]. The bladder and bowel dyssynergia that occurs in patients with spinal cord injury [34] suggests that co-ordination of external anal sphincter relaxation with colorectal propulsion is influenced by centres rostral to the spinal cord, most likely in the cortex and pons.
The gastro-colic reflex, through which colonic activity is enhanced at the time of a meal or soon after, was absent in SCI subjects, while it was present in uninjured subjects in the same study [36]. A later study also reported that a gastro-colic reflex was not seen in the rectosigmoid region in SCI individuals, but that a reflex was seen more proximally, in the descending colon [41]. This suggests that the gastro-colic reflex may influence more proximal regions through the vagus nerve, while the pathways that increase contractile activity in the distal colon and rectum travel in the spinal cord and pelvic nerves.
Gastrointestinal disturbances caused by neurogenic bowel were more often seen in patients with motor complete SCI and injury above about T5 to 7; 67% of those patients suffered from constipation as well as 85% had some level of incontinence [42, 43]. A possible reason that SCI above about T5-7 causes greater problems is that these individuals either cannot, or have reduced ability to, voluntarily contract abdominal muscles. For lesions below T7, patients can increase intra-abdominal pressure by contracting the abdominal wall [44]. Although this assists in activating colorectal propulsion, there is still dyssynergia, with the external anal sphincter commonly contracting when the intra-abdominal pressure is increased [44].
The deduction that the integrity of co-ordinated colorectal propulsive reflex activity is dependent on signals from the defecation centre is consistent with experimental studies in spinal injured rats that show that ghrelin receptor agonists, which are specific stimulants of the defecation centres cause propulsion of the colorectal contents after SCI [45].
Long-term consequences
The frequency and severity of constipation-related symptoms and their impact on quality of life increase with time since injury [46,47,48], although physiological investigations do not indicate a loss of colon function (see below). The changes for patients includes an increase in the time spent at defecation, the requirement for digital anorectal stimulation at least once every week, an increase in the number who report an impact of colorectal dysfunction on quality of life and an increase in the number of respondents who defecated less than every second day. There was, however, a decrease in fecal incontinence [47].
However, there was no change in the gastrointestinal transit time nor in the incidence of megacolon in patients who were examined 1 year post injury, with a second analysis 10 years post injury, nor in a second group that were on average 19 years post injury when first examined and an average of 29 years post injury at retest [49]. These results are in agreement with an earlier study that found no change in fecal incontinence score with either duration of injury or age for SCI patients, although there was an increase in laxative use [46]. Another questionnaire based cross-sectional study of spinal cord-injured individuals showed that a high level of cord lesion, completeness of cord injury and longer duration of injury (≥ 10 years) can predict the severity of bowel dysfunction [12].
Chronic abdominal pain was found to affect over 30% of paraplegic respondents to a longitudinal postal survey with a mean time post injury of 30 years [50]. The abdominal pain was related to constipation with a higher frequency of self-reported constipation. Full bladder and bladder infection often increased abdominal pain or discomfort. The study also found that opioids, which could exacerbate constipation, were the most common treatment reported for abdominal pain.
Bowel management
Conservative management includes mechanical means of aiding emptying (abdominal massage, digital rectal stimulation, manual evacuation, trans-anal irrigation), use of chemical agents (oral laxatives, stool softeners, colonic stimulants, bulk forming agents, bisacodyl suppositories), changes in diet (high dietary fibre) and pharmacological agents, notably anticholinesterases [51,52,53]. Most people with SCI develop a bowel routine using more than one conservative management procedure [44, 52].
Evaluation of procedures in controlled, adequately powered trials has been inadequate [52]. Nevertheless, there is some evidence to support these approaches, particularly in combination, and about two thirds of patients find bowel routines that improve their situation [53].
Where these conservative measures prove inadequate, surgical treatments are available, that include sacral nerve stimulation or sacral anterior roots stimulation (SARS) with implanted stimulation systems, creating a colostomy, or providing a surgical entry to allow bowel irrigation (Malone procedure). The SARS procedure involves placing stimulating electrodes on the ventral roots at S2-S4 and severing the dorsal roots. The method is commonly used to treat neurogenic urinary bladder disorders, but has also been used to treat bowel dysfunction in people living with spinal cord injury [54]. In a study of over 200 patients, SARS increased the frequency of defecation, reduced bowel emptying times and reduced the use of laxatives, suppositories and digital evacuation [54].
Colokinetic drugs
Some SCI patients have had success in relieving constipation with 5-HT receptor stimulants, tegaserod and cisapride, but these colokinetics have been removed from the market due to side effects [52]. The other colokinetic protocol is to use the anti-cholinesterase, neostigmine plus an antagonist (glycopyrrolate) that is used to limit the effects of increased acetylcholine availability on the heart [55, 56]. In animal studies neostigmine has been found to enhance contractions in the colorectum, but the contractions are commonly not propulsive.
More could probably be done to investigate further colokinetic drugs that do enhance colorectal propulsive activity. Prucalopride, also a 5-HT agonist, is approved to treat constipation in Europe [57]. It had earlier been trialled in SCI [58], but has not become a regularly used drug treatment.
As mentioned above, in human the co-ordinated propulsive activity of the colorectum depends on both the ENS and the spinal defecation centres. Thus, agents that activate the spinal defecation centres may be useful colokinetics. The only class of drug that has been identified that stimulates the defecation centre to cause co-ordinated propulsion is ghrelin receptor agonists that cross the blood-spinal cord barrier [59,60,61]. These compounds also cause defecation in human volunteers and humans with SCI [62,63,64]. Animal studies show that an anti-cholinesterase enhances colorectal contractions, but these contractions are poorly propulsive, whereas ghrelin receptor agonists elicit propulsive contractions.
Discussion
Injury to the spinal cord that is located below the cortical centres for voluntary control of continence and defecation, and below the ponto-medullary centres for co-ordination of bowel emptying and external anal sphincter relaxation, causes loss of colorectal control (Fig. 1). Autonomic neural circuits for co-ordinated movement of the bowel are also located at the sacral level of the spine (spinal defecation centre) and in the bowel wall (the enteric nervous system). These centres and their local connections constitute the involuntary parts of the autonomic defecation control system. The somatic circuits that control the external sphincter are directed from the cortex and pons. Because the spinal and enteric autonomic circuits that control the distal colon and rectum remain intact when injury is above the sacral level, pharmaceutical agents can be used to trigger or augment their function, although it needs to be considered that denervation can lead to changes in neural properties, for example, denervation supersensitivity [40, 65], meaning that sensitivities to drugs may differ between patients and uninjured controls. However, there is no available pharmacological method to relax the eternal sphincter. Thus, new drug therapies will most likely be adjuncts to non-pharmacological methods to aid bowel function.
References
Widerström-Noga EG, Felipe-Cuervo E, Broton JG, Duncan RC, Yerierski RP. Perceived difficulty in dealing with consequences of spinal cord injury. Arch Phys Med Rehabil. 1999;80:580–6.
Burns AS, St-Germain D, Connolly M, Delparte JJ, Guindon A, Hitzig EL, et al. Phenomenological study of neurogenic bowel from the perspective of individuals living with sinal cord injury. Arch Phys Med Rehabil. 2015;96:49–55.
Braaf S, Lennox A, Nunn A, Gribble B. Social activity and relationship changes experienced by people with bowel and bladder dysfunction following spinal cord injury. Spinal Cord. 2017;55:679–86.
Glickman S, Kamm MA. Bowel dysfunction in spinal-cord-injury patients. The Lancet. 1996;347:1651–3.
Lynch AC, Antony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury. Spinal Cord. 2001;39:193–203.
Lynch AC, Frizelle FA. Colorectal motility and defecation after spinal cord injury in humans. Prog Brain Res. 2006;152:335–43.
Ng C, Prott G, Rutkowski S, Li Y, Hansen R, Kellow J, et al. Gastrointestinal symptoms in spinal cord inury: Relationships with level of injury and psychologic factors. Dis Colon Rectum. 2005;48:1562–8.
Levi R, Hultling C, Nash MS, Seiger A. The Stockholm spinal cord injury study: 1. Medical problems in a regional SCI population. Spinal Cord. 1995;33:308–15.
Han TR, Kim JH, Kwon BS. Chronic gastrointestinal problems and bowel dysfunction in patients with spinal cord injury. Spinal Cord. 1998;36:485–90.
Snoek GJ, Ijzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42:526–32.
Krogh K, Perkash I, Stiens SA, Biering-Sorensen F. International bowel function extended spinal cord injury data set. Spinal Cord. 2009;47:235–41.
Liu C-W, Huang C-C, Chen C-H, Yang H-J, Chen T-W, Huang M-H. Prediction of severe neurogenic bowel dysfunction in persons with spinal cord injury. Spinal Cord. 2010;48:554–9.
Dubrovsky B, Filipini D. Neurobiological aspects of the pelvic floor muscles involved in defecation. Neurosci Biobehav Rev. 1990;14:157–68.
Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4:1339–68.
Vizzard MA, Brisson M, de Groat WC. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res. 2000;299:9–26.
Sanger GJ, Furness JB. Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2016;19:38–48.
Blok BFM, Holstege G. Two pontine micturition centers in the cat are not interconnected directly: implications for the central organization of micturition. J Comp Neurol. 1999;403:209–18.
Rouzade-Dominguez M-L, Pernar L, Beck S, Valentino RJ. Convergent responses of Barrington’s nucleus neurons to pelvic visceral stimuli in the rat: a juxtacellular labelling study. Eur J Neurosci. 2003;18:3325–34.
Blok BFM, Holstege G. Central nervous system control of micturition in cats and humans. Behav Brain Res. 1998;92:119–25.
Pavcovich LA, Yang M, Miselis RR, Valentino RJ. Novel role for the pontine micturition center, Barrington’s nucleus: evidence for coordination of colonic and forebrain activity. Brain Res. 1998;784:355–61.
Loewy AD, Saper CB, Baker RP. Descending projections from the pontine micturition center. Brain Res. 1979;172:533–8.
Nuding SC, Nadelhaft I. Bilateral projections of the pontine micturition center to the sacral parasympathetic nucleus in the rat. Brain Res. 1998;785:185–94.
Verstegen AMJ, Vanderhorst V, Gray PA, Zeidel ML, Geerling JC. Barrington’s nucleus: Neuroanatomic landscape of the mouse “pontine micturition center”. J Comp Neurol. 2017;525:2287–309.
Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–66.
Holmes GM, Martau JM, Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Nucleus raphe obscurus (nRO) regulation of anorectal motility in rats. Brain Res. 1997;759:197–204.
Hermann GE, Bresnahan JC, Holmes GM, Rogers RC, Beattie MS. Descending projections from the nucleus raphe obscurus to pudendal motoneurons in the male rat. J Comp Neurol. 1998;397:458–74.
Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. J Comp Neurol. 1996;375:502–17.
Kuzuhara S, Kanazawa I, Nakanishi T. Topographical localization of the Onuf’s nuclear neurons innervating the rectal and vesical striated sphincter muscles: a retrograde fluorescent double labeling in cat and dog. Neurosci Lett. 1980;16:125–30.
Holstege G, Tan J. Supraspinal control of motoneurons innervating the striated muscles of the pelvic floor including urethral and anal sphincters in the cat. Brain. 1987;110:1323–44.
Gerrits PO, JAML Sie, Holstege, Motoneuronal G. Location of the external urethral and anal sphincters: a single and double labeling study in the male and female golden hamster. Neurosci Lett. 1997;226:191–4.
Broens PMA, Penninckx FM, Ochoa JB. Fecal continence revisited: the anal external sphincter continence reflex. Dis Colon Rectum. 2013;56:1273–81.
Beckel JM, Holstege G Neuroanatomy of the lower urinary tract. In: Andersson K-E & Michel MC (eds), Urinary tract. Springer, Berlin; 2011. p. 99–116.
Shafik A. Recto-colic reflex: role in the defecation mechanism. Int Surg. 1996;81:292–4.
Lynch AC, Anthony A, Dobbs BR, Frizelle FA. Anorectal physiology following spinal cord injury. Spinal Cord. 2000;38:573.
Norton L. Spinal cord injury. Australia (2007–08): Australian institute of health and welfare; 2010.
Aaronson MJ, Freed MM, Burakoff R. Colonic myoelectric activity in persons with spinal cord injury. Dig Dis Sci. 1985;30:295–300.
Schuster MM, Hookman P, Hendrix TR, Mendelhoff AI. Simultaneous manometric recording of internal and external anal sphincteric reflexes. Bull John Hopkins Hosp. 1965;116:79–88.
De Groat WC, Krier J. The sacral parasympathetic reflex pathway regulating colonic motility and defaecation in the cat. J Physiol. 1978;276:481–500.
MacDonagh R, Sun WM, Thomas DG, Smallwood R, Read NW. Anorectal function in patients with complete supraconal spinal cord lesions. Gut. 1992;33:1532–8.
Tankisi H, Pugdahl K, Rasmussen MM, Clemmensen D, Rawashdeh YF, Christensen P, et al. Pelvic floor electrophysiology in spinal cord injury. Clin Neurophysiol. 2016;2016:2319–24.
Fajardo NR, Pasiliao R-V, Modeste-Duncan R, Creasey G, Bauman WA, Korsten MA. Decreased colonic motility in persons with chronic spinal cord injury. Am J Gastroenterol. 2003;98:128–34.
Vallès M, Vidal J, Clavé P, Mearin F. Bowel dysfunction in patients with motor complete spinal cord injury: Clinical, neurological, and pathophysiological associations. Am J Gastroenterol. 2006;101:2290–9.
Ozisler Z, Koklu K, Ozel S, Unsal-Delialioglu S. Outcomes of bowel program in spinal cord injury patients with neurogenic bowel dysfunction. Neural Regener Res. 2015;10:1153–8.
Hughes M. Bowel management in spinal cord injury patients. Clin Colon Rectal Surg. 2014;27:113–5.
Ferens DM, Habgood MD, Saunders NR, Tn YH, Brown DJ, Brock JA, et al. Stimulation of defecation in spinal cord-injured rats by a centrally acting ghrelin receptor agonist. Spinal Cord. 2011;49:1036–41.
Lynch AC, Wong C, Anthony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury: a description of bowel function in a spinal cord-injured population and comparison with age and gender matched controls. Spinal Cord. 2000;38:717–23.
Faaborg PM, Christensen P, Finnerup N, Lauberg S, Krogh K. The pattern of colorectal dysfunction changes with time since spinal cord injury. Spinal Cord. 2008;46:234–8.
Finnerup NB, Faaborg P, Krogh K, Jensen TS. Abdominal pain in long-term spinal cord injury. Spinal Cord. 2008;46:198–203.
Faaborg PM, Christensen P, Rosenkilde M, Laurberg S, Krogh K. Do gastrointestinal transit times and colonic dimensions change with time since spinal cord injury? Spinal Cord. 2011;49:549–53.
Nielsen SD, Faaborg PM, Christensen P, Krogh K, Finnerup NB. Chronic abdominal pain in long-term spinal cord injury: a follow-up study. Spinal Cord. 2017;55:290–3.
Krassioukov A, Eng JJ, Claxton G, Sakakibara BM, Shum S. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord. 2010;48:718–33.
Coggrave M, Norton C, Cody JD. Management of faecal incontinence and constipation in adults with central neurological diseases (Review). In: Group CI, editor. Cochrane Database of Systematic Reviews. Wiley, Hoboken, NJ, USA; 2014. p. 1–61.
Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop. 2015;6:24–33.
Rasmussen MM, Kutzenberger J, Krogh K, Zepke F, Bodin C, Domurath B, et al. Sacral anterior root stimulation improves bowel function in subjects with spinal cord injury. Spinal Cord. 2015;53:297–301.
Korsten MA, Rosman AS, Ng A, Cavasoglu E, Sprungen AM, Radulovic M, et al. Infusion of neostigmine–glycopyrrolate for bowel evacuation in persons with spinal cord injury. Am J Gastroenterol. 2005;100:1560–5.
Rosman AS, Chaparala G, Monga A, Sprungen AM, Bauman WA, Korsten MA. Intramuscular neostigmine and glycopyrrolate safely accelerated bowel evacuation in patients with spinal cord injury and defecatory disorders. Dig Dis Sci. 2008;53:2710–3.
Sajid MS, Hebbar M, Baig MK, Li A, Philipose Z. Use of prucalopride for chronic constipation: a systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016;22:412–22.
Krogh K, Jensen MB, Gandrup P, Lauerberg S, Nilsson J, Kerstens R. Efficacy and tolerability of prucalopride in patients with constipation due to spinal cord injury. Scand J Gastroenterol. 2002;37:431–6.
Shimizu Y, Chang EC, Shafton AD, Ferens DM, Sanger GJ, Witherington J, et al. Evidence that stimulation of ghrelin receptors in the spinal cord initiates propulsive activity in the colon of the rat. J Physiol (London). 2006;576:329–38.
Pustovit RV, Callaghan B, Kosari S, Rivera LR, Thomas H, Brock JA, et al. The mechanism of enhanced defecation caused by the ghrelin receptor agonist, ulimorelin. Neurogastroenterol Motil. 2014;26:264–71.
Naitou K, Mamerto TP, Pustovit RV, Callaghan B, Rivera LR, Chan AJ, et al. Site and mechanism of the colokinetic action of the ghrelin receptor agonist, HM01. Neurogastroenterol Motil. 2015;27:1596–603.
Ejskjaer N, Dimcevski G, Wo J, Hellestrom PM, Gormsen LC, Sarosiek E, et al. Safety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2010;22:1069–e281.
Ellis AG, Zeglinski PT, Brown DJ, Frauman AG, Millard M, Jurness JB. Pharmacokinetics of the ghrelin agonist capromorelin in a single ascending dose Phase-I safety trial in spinal cord-injured and able-bodied volunteers. Spinal Cord. 2015;53:103–8.
Acosta A, Camilleri M, Busciglio I, Boldingh A, Nelson AD, Burton D. Short-term effects of relamorelin on descending colon motility in chronic constipation: a randomized, controlled trial. Dig Dis Sci. 2016;61:852–60.
Pustovit RV, Callaghan B, Ringuet MT, Kerr NF, Hunne B, Smyth IM, et al. Evidence that central pathways that mediate defecation utilize ghrelin receptors but do not require endogenous ghrelin. Physiol Rep. 2017;5:e13385.
Acknowledgements
We thank Billie Hunne for assistance in creating Fig. 1. This work is supported by the Transport Accident Commission, through the Institute for Safety, Compensation and Recovery Research (grant number N-13-085), and the National Health and Medical Research Council of Australia (project grant number 1079739). Some of the pharmacological investigations are supported by Takeda Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Callaghan, B., Furness, J.B. & Pustovit, R.V. Neural pathways for colorectal control, relevance to spinal cord injury and treatment: a narrative review. Spinal Cord 56, 199–205 (2018). https://doi.org/10.1038/s41393-017-0026-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-017-0026-2
This article is cited by
-
Region-specific remodeling of the enteric nervous system and enteroendocrine cells in the colon of spinal cord injury patients
Scientific Reports (2023)
-
Neural signalling of gut mechanosensation in ingestive and digestive processes
Nature Reviews Neuroscience (2022)
-
Diagnosis of colonic dysmotility associated with autonomic dysfunction in patients with chronic refractory constipation
Scientific Reports (2022)
-
Understanding the physiology of human defaecation and disorders of continence and evacuation
Nature Reviews Gastroenterology & Hepatology (2021)
-
Effects of highly selective sympathectomy on neurogenic bowel dysfunction in spinal cord injury rats
Scientific Reports (2021)