Abstract
Study Design
Psychometric.
Objectives
Assess the validity of bioimpedance-based measures of fat-free mass (FFM) in acute SCI and of current definitions of obesity based on body mass index (BMI).
Setting
Australia.
Methods
All admissions within eight weeks of a new traumatic SCI were screened. 29% were eligible. 71% of those consented. Twenty participants (18 male) completed deuterium dilution (DD) and bioimpedance-based measurements of FFM. Thirteen also underwent dual-energy x-ray absorptiometry. Strength of relationships and agreement were examined using Lin’s concordance coefficient and limits of agreement analysis, respectively. Sensitivity and specificity were calculated for three BMI cutoffs for obesity, using percentage fat mass (%FM) obtained from DD as reference.
Results
Median time since injury was 41 days (IQR 28–48). FFM from DD and DXA were highly correlated but not identical. Concordance and agreement between DD and seven bioimpedance-based predictive equations are presented. The best-fitting equation demonstrated a low bias (+0.6 kg) and moderate dispersion (±5.2 kg). The cutoff for overweight in able-bodied people (BMI ≥25 kg/m2) provided sensitivity of 43.8%, compared to 25% for the cut-off for obesity (BMI ≥30 kg/m2). FM from bioimpedance gave the highest sensitivity (88.9%).
Conclusions
BMI demonstrates poor specificity to classify obesity in acute SCI. Present findings support the utility of bioimpedance-based measurements for estimating FFM in acute SCI for group comparisons. These results are generalizable to traumatic SCI 4–8 weeks post injury; however, the present data reflect a high proportion of high cervical injuries. Further research is indicated to establish validity for assessment of individuals and for longitudinal monitoring.
Sponsorship
The present study was funded by a grant from the Institute for Safety, Compensation and Recovery Research (ISCRR Project #NGE-E-13-078). M Panisset was supported by an Australian Postgraduate Award. K Desneves was supported by the Austin Medical Research Foundation.
Similar content being viewed by others
Introduction
The initial inflammatory response to traumatic injury triggers a catabolic process, whereby protein is scavenged from muscle tissues to support synthesis of critical visceral proteins. After spinal cord injury (SCI), initial neurologic and spinal shock are characterized by flaccid paralysis and physical activity is substantially limited. This combination of catabolism, paralysis and decreased activity precipitates rapid atrophy of muscle tissue. Loss of 33% of thigh muscle area occurs by six weeks post injury after incomplete SCI [1]. Further atrophy, up to 27–56%, occurs from 6–24 weeks in complete SCI [2].
Atrophy is accompanied by increasing adiposity over time. Changes in body composition contribute to cardiometabolic disease and associated morbidities, such as hyperinsulinemia, diabetes mellitus and dyslipidemia [3, 4]. Ten percent of individuals with chronic SCI have a moderate to high risk of long-term cardiac events, with obesity being the most prevalent cardiometabolic risk factor [3, 5].
Thus, effects from dietetic management in acute SCI may have lasting consequences. Nutritional protein supplementation is required to support tissue healing post injury. Poor nutritional status is associated with increased risk of pressure injuries, infections, respiratory complications, increased length of stay and early mortality [6]. On the other hand, one study reported SCI patients in sub-acute rehabilitation (mean 65.5 ± 12.5 days post injury) gained a mean 1.7 kg/week on unrestricted diets [7]. Monitoring changes in body composition could prevent both malnutrition and overfeeding. Weight change alone is not sufficient for this purpose, because weight loss from muscle atrophy is likely to obscure increases in fat mass (FM).
Assessment of body composition
Body mass index (BMI), calculated from height and weight (kg/m2) is widely used to classify obesity. However, the sensitivity of BMI to distinguish obese from non-obese paraplegics is low (20%) [8].
Dual-energy x-ray absorptiometry (DXA), primarily designed to assess bone density, also provides measures of FM and fat-free mass (FFM). DXA is precise and reliable, although its accuracy is limited in individuals with high FM or very low fat-free mass [9], as may occur after SCI. Furthermore, many DXA scanning beds are inaccessible for wheelchair users. Thus, a more practical alternative to measure body composition in acute SCI is needed.
Single frequency bioelectrical impedance analysis (SF-BIA) has been investigated for this purpose. Accurate measures of FFM, largely comprised of muscle tissue [10], are derived from total body water (TBW), assuming a hydration fraction for FFM of 0.732 [11]. SF-BIA utilizes a portable device to measure the body’s impedance to an alternating electrical current, typically at 50 kHz (BIA50), sent between dermal electrodes [12]. TBW is calculated from the measured impedance using the equation:
Where ρ is the specific resistivity of body fluids (ohm.cm), L is the distance (cm) between electrodes (or surrogate measurement such as height) and Z is the measured impedance (ohm). Commonly, resistance (R) is substituted for impedance. (17)
SF-BIA has been validated for longitudinal assessment of able-bodied subjects (BMI range 16–34 kg/m2) [12]. Empirical regression equations incorporating resistance quotient (L2/R) and other predictors such as age, sex and weight, have been developed in other neurological conditions [13]. However, these equations are recognized to be population-specific [12] and to date none have been validated in acute SCI.
Bioimpedance spectroscopy
TBW comprises intracellular water (ICW) and extracellular water (ECW). It has been hypothesized that the accuracy of single frequency BIA50 to predict TBW can be affected by changes in ECW, which is often increased in SCI [14]. Bioimpedance spectroscopy (BIS) measures impedance across a continuum of frequencies (typically 3–1200 kHz), and is thought to provide increased precision in populations where ECW is increased [15]. BIS has shown promise for assessing ECW and ICW in chronic SCI [8], and may prove useful in acute SCI.
Isotope tracer dilution is the reference method for measuring TBW; however, tracer dilution methods are costly and require specialized facilities and expertize. Thus, dilution methods are often used to validate other, more practical, methods. The aim of this study was to examine the validity of bioimpedance methods (BIA and BIS) to assess FFM in acute SCI. TBW measures derived from deuterium tracer dilution were compared to estimates of FFM from BIS and relevant BIA-based predictive equations extracted from the literature (Table 1).
It has been suggested in previous studies that more stringent BMI cutoffs be used to classify obesity in chronic SCI ([16, 17] Laughton, 2009 #386;Silveira, 2017 #359). Thus, a secondary aim was to assess the validity of BMI for classifying obesity in acute SCI using percentage FM (%FM) values from tracer dilution as reference.
Methods
Participants
This study was approved by the Austin Health Human Research Ethics Committee (ANZCTR Trial# ACTRN12615000178549). Patients admitted to the Austin Health Victorian Spinal Cord Service within 8 weeks of traumatic SCI above T12 were screened. Medical clearance was sought from their spinal physician and written informed consent was obtained.
Deuterium dilution
TBW was calculated using the deuterium dilution (DD) data from a doubly-labelled water (DLW) dilution protocol, as part of a parallel research programme. The DLW, comprising 99% 2H2O (deuterium) and 97% 18O in solution of normal water for a total density of 1.11 g/mL, was administered orally at a dose rate of 0.2 g/kg [18]. This dose was adjusted to 0.21 g/kg for a supplementary batch of DLW that comprised 95% 2H2O and 97% 18O (density 1.2 g/mL). Doses were prepared by the hospital pharmacy. Exact dose ingested was determined by the difference of the initial weight of solution and cup before minus the weight of the cup and any residual after ingestion.
Baseline urine samples were collected early in the day, prior to administration of the labelled water (Day 0) to determine any background level of isotopes. Subsequent samples were collected from the participant’s catheter port via syringe twice on day 1, after 3 and 4 h, and in the morning on post-dose days 2, 5, 6, 8, 9, 12 and 13. Samples were frozen at −20 °C and, at the conclusion of the study, shipped to the Children’s Nutrition Research Centre at the University of Queensland for analysis using an isotope ratio mass spectrometer (Isoprime Dual Inlet IMRS-IonVantage Software, Isoprime, Manchester, UK). TBW was calculated using standard equations [18].
Bioimpedance analysis
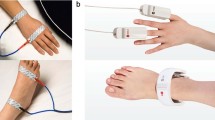
Bioimpedance measurements were taken in the morning on fasting participants, in climate-controlled in-patient rooms, using a four terminal BIS instrument (model SFB7, ImpediMed Ltd., Brisbane, Australia). Participants hydrated normally overnight. To minimize fluctuations in body fluid distribution, all participants were measured after lying recumbent at least 60 min. Current drive Ag-AgCl EKG-style electrodes were placed at dorsal ankles and wrists. The SFB7 records impedance (Z) and its components, resistance (R) and reactance (Xc), at each of 256 logarithmically spaced frequencies in the range 3–1000 kHz. Three replicate measurements were recorded, downloaded from the instrument to a laptop computer and analyzed using Bioimp software version v4.18.0 (ImpediMed Ltd., Brisbane, QLD, Australia). Default analysis parameters set by the manufacturer were employed: frequency window 10–500 kHz, zero noise rejection, automatic correction for high frequency capacitive effects and time delay correction. Data were cleaned and valid replicate measurements were averaged.
Bioimp software uses regression procedures to fit the recorded data (R and Xc) to the semi-circular Cole model of the impedance properties of biological tissues [19], to derive the extrapolated resistance at zero frequency (R0) and at infinite frequency (R∞). R0 is inversely proportional to ECW; R∞ is inversely proportional to TBW. ECW and TBW, and by difference ICW, can be predicted from R0 and R∞ according to Hanai mixture theory [20]. This method requires the use of apparent resistivity coefficients for ECW and ICW. These have not been determined for SCI patients; consequently values were taken from the largest data set published to date [21].
FFM was predicted from BIS data (BISLCW) [21]. BIS uses fundamental prediction methods (mixture theory) rather than specific empirical prediction equations, with assumed coefficients for body density, body proportion and fluid resistivities [22]. The assumed values for body density was 1.05 g/mL and for hydration fraction was 0.732 [11]. In addition, resistance data were recorded at discrete single frequencies for calculation of BIA-based equations (Table 1).
Total Body DXA
DXA whole body fan beam scans were performed on a GE-Lunar Prodigy scanner (GE Healthcare, Cleveland, USA. enCORE software v13.60, Madison, USA) within 24 h of bioimpedance assessment. No participant exercised within 90 min prior to the scan. Although participants were not required to fast, DXA scans occurred just before lunchtime. Standardized segmentation was performed by one investigator. One participant had a hip implant. This area was excluded and values from the opposite limb represented both sides.
Anthropometry
Body weight was measured to the nearest 100 g on either Gludmann or Wedderburn Rinstrum R320 hoist scales or Wedderburn Model No 2100 platform scales the day before study commencement. Height was measured in supine to the last 0.5 cm, using portable metal bookends and a rigid tape measure.
Limiting of bias
While there was no blinding explicit in the study design, each type of measurement was conducted by separate researchers, so that no measurement was taken with knowledge of values obtained with different instruments.
Statistical analysis
Statistical analyses were performed using Medcalc (MedCalc Software bvba, Ostend Belgium). Descriptive results are expressed as median and interquartile range (IQR). P < 0.05 was considered statistically significant. Lin’s concordance coefficient (CCC) was used to evaluate concordance, precision and accuracy of FFM from each method compared to DD. CCC takes into account the correlation between measurements and the deviation from the 45° line of identity by calculating a bias correction factor to the Pearson correlation coefficient measures how far the best-fit line deviates from the 45° line through the origin, and is a measure of accuracy [23]. Concordance was considered good if r > 0.6 and high if r > 0.8.
Bland-Altman limits of agreement (LOA) plots are a graphical representation of the between-method differences, plotted along the y axis, and the assumed true value plotted along the x axis, calculated as the average of measures [24].
Sensitivity and specificity were calculated for three BMI cutoffs: the World Health Organisation (WHO) classification for overweight and for obese [25] and a previously-recommended lower cutoff for SCI (BMI ≥ 22 kg/m2) [16], using values for %FM [27] obtained from DD as a reference (Supplementary Table). A sensitivity analysis of %FM from the best-fitting bioimpedence-based prediction equation was also conducted.
Results
Participant characteristics
86 patients met the inclusion criteria (76% of all admissions) between November 2013 and May 2015. Reasons for exclusion included age <18 (n = 5), death (n = 4), language barrier (n = 1) and concurrent illness (n = 22). Data were not collectable from twenty participants due to interruption in the DLW supply, short length of stay, cytotoxicity or lack of an indwelling catheter. 29% of all admissions were recruited, 71% of whom consented.
Descriptive characteristics are presented in Table 2. Twenty participants gave consent and completed both DLW and bioimpedance assessments. Thirteen participants completed DXA measurements, but one of those did not complete DLW. Median age was 42.5 years (IQR 24–61 years). Median time since injury was 41 days (IQR 28–48 years). Median BMI at baseline was 24.9 (IQR 21–28 years). Five participants had complete injuries (two tetraplegia). Fifteen had incomplete injuries, (14 tetraplegia). Participants were Caucasian males, except for two females (Asian and Caucasian).
Validity of FFM from DXA
Predicted FFM from all methods is displayed in Fig. 1. Table 3 presents Lin’s concordance coefficient (CCC), Pearson’s correlation coefficient (r, a measure of precision) and the bias correction factor (Cb) for all methods compared to DD. The measurements of FFM from DD and DXA showed high concordance (rc = 0.88), however the Bland-Altman plot shows the bias between methods was 1.7 kg, with LOA −5.1 to 8.5 kg (Fig. 2). This equated to an underestimation of FFM by 2.9% using DXA, with limits of agreement (LOA) ±11.5%, indicating decreased accuracy compared to some bioimpedance-based predictions as discussed below.
Box plots show the median of each measure (centre line of the box). The limits of the box represent the 25th and 75th percentiles of the group data. Whiskers show the range of the data, except when there are outliers, which are represented by outlying points. Key (references): BIAK50 [26]; BIAB50 [14]; BIADesport [13]; BIASegal [29]; BIAv50 [27]; BIAV100 [27]; BIALukaski [28]; BISLCW [19]
Valididty of bioimpedance analysis
Of all the predictive equations tested, the population-specific BIAK50 had the best fit (Table 3) [28]. The Bland-Altman plot (Fig. 3a) shows a mean bias of 0.6 kg and LOA ±5.2 kg. However, the slope of the regression line was significantly different from 0, indicating systematic error; BIAK50 increasingly underestimated FFM as the value of FFM increased.
The BIAB50 equation showed the next best fit (Fig. 3b), with double the bias of BIAK50 [14]. BIAB50 systematically overestimated FFM as values of FFM increased and underestimated FFM where FFM values were lower.
FFM predicted from BIAV100 and BIAV50 had very good concordance, precision and accuracy (Table 3) [29]. Unlike BIAK50 and BIAB50, neither BIAV100 nor BIAV50 displayed systematic bias, but the bias and LOA were much larger (Fig. 3c, d). BIASegal and BIALukaski were not a good fit (Fig. 3e, f) [30, 31].
BIADesport, developed in patients with ALS [13], was the only equation that incorporated an anthropometric measure (triceps skin fold). Although concordance with DD was very good and the mean bias was small (1.2 kg), the LOA were unacceptably wide (Fig. 3g).
While predictions of FFM by BISLCW [19], also showed high concordance with DD, the Bland-Altman plot depicts a bias of 4.3 kg (Fig. 3h). BISLCW increasingly underestimated FFM as values decreased.
Classifying obesity in acute SCI
The prevalence of obesity, as determined by %FM, was 45%. Results from the sensitivity analysis are presented in Table 4. Although BMI is the most widely used classification system for obesity in SCI [17], we were unable to identify a suitable BMI cutoff for classifying obesity in this sample. DXA %FM had perfect sensitivity and specificity, while %FM from BIAK50 showed perfect specificity and high sensitivity (88.9%).
Discussion
This study evaluated the validity of several published equations estimating FFM from BIA measurements, demonstrating very good concordance (rc 0.82–0.96). Two equations (BIASegal and BIALukaski) based on large samples of able-bodied people demonstrated poor accuracy in this sample, confirming accepted knowledge that predictive equations from bioimpedance are population-specific [12].
Previously, BIAV100 was able to predict TBW in persons with chronic SCI with minimal bias (0.76 L) and moderate dispersion (LOA −2.9 to 4.4 L) [33]. However, BIAV100 showed an overestimation bias and larger dispersion in the present study, despite a similar sample size [33]. The reasons for this difference are unclear. The previous sample had a slightly higher ratio of females to males (1/3) and a slightly higher median BMI (26.9 ± 4.4). The ratio of paraplegia to tetraplegia was not reported but was likely to have also been higher, as our sample comprised mostly high tetraplegia. Another probable source of discrepancy was time post injury; the previous sample was at least four months post injury, but the median time post injury was not reported and may have been considerably longer.
BIADesport and BIAB50 systematically overestimated FFM in individuals with higher FFM in the present study [13, 14]. BIADesport was developed in patients with ALS; the extent of the atrophy in that patient cohort would have progressed more than in this acute SCI sample. Furthermore, in cases of paraplegia, a large portion of the body may not be affected, whereas ALS affects the whole body.
BIAB50 was derived in a sample of 62 able-bodied subjects, then cross-validated in chronic SCI, overestimating FFM by 2.3 kg compared to DD [14]. The present study is the first to evaluate BIAB50 in acute SCI, demonstrating very good concordance and a low underestimation bias, however the dispersion was ±7.6 kg. The previous chronic SCI sample comprised only adults with complete paraplegia [14], whereas the present sample was predominantly comprised of incomplete tetraplegia, with considerably higher FFM than the BIAB50 sample (58.4 ± 10 kg compared to 44 ± 11 kg). This is a likely source of discrepancy, given the systematic overestimation observed when FFM increased.
The equation providing the best fit for this sample was BIAK50, which overestimated FFM by only 600 g and provided the tightest LOA of all the bioimpedance-based measures (±5.2 kg). Previous reports that BIAK50 estimates of FM correlated moderately with DXA in men (r = 0.722, P = 0.043) but not in women (r = 0.484, P = 0.224) with chronic SCI and that overall precision was poor (total error 6.7% in men and 8.1% in women) [34]. The poor precision in females should be noted, as the current sample included only two females. Other differences between the two samples include recency of injury and high proportion of incomplete tetraplegics in the current study, compared to chronic paraplegic athletes in the former. Although the high concordance with DD lends some support to the construct validity of BIA in general, these data cannot yet validate BIAK50 for assessment of individuals with acute SCI. A larger sample size might provide tighter LOA, and further testing to validate this equation is warranted.
Although BIS theoretically provides a better estimate of TBW and hence FFM, the purported improvement of BIS over BIA has been reported to be small [35]. In the present study, BISLCW performed no better than single frequency predictors. This may be due to the use of resistivity coefficients for healthy rather than SCI individuals. Furthermore, the body proportion factor was also derived from healthy individuals and may not be appropriate for individuals with substantial atrophy.
DXA is widely regarded as an alternative to costly and logistically difficult dilution methods and has the advantage of simultaneously assessing multiple body compartments (FM, FFM and bone mineral). Although highly correlated with DD in this sample, DXA underestimated FFM by 2.9% with wide LOA (±11.5%). These findings indicate that DXA and DLW methods may be considered interchangeable within ±2.9% error margin for group means. Although LOA may be expected to decrease with a larger sample, these results are consistent with literature questioning the accuracy of DXA for measurements of body composition in individuals with extreme atrophy [9].
Classification of obesity
Previous proposals that a more stringent BMI cut-off (≥22 kg/m2) should be used to classify obesity in chronic SCI [4, 24, 32], however this low cut-off produced false positives in 40% of the present sample. Obesity classified using BIAK50 was more much sensitive (88.9%) than BMI. This is superior to previous reports that BIAB50 had 68.4% sensitivity, outperforming BMI (20% sensitivity) to identify obesity in chronic paraplegia [14]. Although DXA is perfectly valid for classifying obesity, BIAK50 is less expensive, clinically feasible and suitable for longitudinal assessments. Further research should endeavour to replicate these findings and to validate the connection between %FM and associated morbidities.
Clinical relevance
The development of an objective bedside measure to quantify body composition in acute SCI may facilitate improvements in patient care. BIA shows utility as a sensitive outcome measure for clinical research. A pilot study on the effects of FES rowing intervention on body composition in people with SCI (>6 mos) was able to detect statistically significant pre-post differences using BIA50 measures of FFM and FM, but not using BMI or waist circumference [36]. The current study supports the utility of BIAK50 for estimating body composition for group studies in acute SCI. These results are generalizable to traumatic SCI (AIS A–D) within 4–8 weeks post injury; however, the present cohort had a potentially greater proportion of high-cervical SCI than other settings. Further data is required to provide greater accuracy and precision in the assessment of individuals. To date, many studies on interventions attempting to slow or reverse muscle atrophy after SCI, such as functional electrical stimulation exercise, have relied on either DXA or time-consuming methods of MR image analysis to quantify changes in skeletal muscle [37, 38]. The validation of BIA measures of FFM in acute SCI may provide greater facility to quantify outcomes in such studies, as well as in clinical practice.
BIA-based estimations of FFM have demonstrated precision (0.9–3.3%) in biannual follow-up measurements in patients with ALS [13]. Given that the maximum weight loss in the present sample during the two-week period was only 2 kg, which is within the standard error of the measurement for DXA soft tissue measures, longitudinal investigations to validate BIA to monitor longitudinal change are warranted.
Validated and clinically practical quantitative measures of FFM might enhance dietetic management in acute SCI through improved accuracy of predictions of nutritional requirements. This, for example, could allow better targeting of protein supplementation to support muscle growth rather than more generalized unwanted weight gain. Skeletal muscle and the liver contribute most significantly to resting metabolic rate (RMR) [10]. FFM accounts for 70–85% of the variance in RMR in non-obese adults [10]. There is limited but consistent evidence that FFM also predicts RMR in people with SCI, accounting for 70% of the variance [8]. Thus, measures of FFM may predict metabolic demands in SCI more accurately than currently utilized methods based largely on height and weight. If so, then this validation of BIA will provide, for the first time, an objective bedside measure to guide dietetic practice in acute SCI.
The main limitation of this study was the small sample size particularly for DXA. This could have contributed to the wide LOA observed for the method comparisons. However, the high cost of isotopes and mass spectrometry analysis precluded the assessment of a larger sample. Notwithstanding the small sample size, the observed LOA were similar in magnitude typical of impedance validation studies in general [37, 39]. Nonetheless, the study helps to clarify the practical feasibility of using this simple objective measurement tool in this challenging population. Future studies that link impedance-derived predictions of body composition to clinical outcome would provide additional support for the clinical utility of the impedance technique.
Conclusions
The present results indicate that BMI has poor specificity to identify obesity in acute SCI. Although DXA produced valid measures of FFM, it was inaccessible to wheelchair users. BIA demonstrated validity to classify obesity in acute SCI, was well tolerated by patients, and practical to implement.
Several predictive equations to estimate FFM were tested. The equation derived and cross-validated in chronic SCI provided the best fit for this acute SCI sample (BIAK50) [28]. BIAK50 showed the best precision and accuracy, although systematic bias with relatively large LOA was evident; replication of these findings is needed and if supported then this impedance protocol could provide an objective simple to use bedside measurement. The high precision and reproducibility of the bioimpedance technique in general lends itself to the measurement of FFM in acute SCI for group comparisons [28]. Larger studies may demonstrate improved accuracy and increased confidence in BIAK50 for the long-term clinical assessment of FFM in SCI individuals.
Validation of a practical and accurate method to quantify body composition could advance the dietetic management in acute SCI. The relationship between FFM and energy requirements [8] and clinical outcomes and/or response to nutritional interventions merits investigation in this patient population.
References
Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–9.
Castro MJ, Apple DF Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol (1985). 1999;86:350–8.
Groah SL, Nash MS, Ward EA, Libin A, Mendez AJ, Burns P, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev. 2011;31:73–80.
Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513–8.
Flank P, Wahman K, Levi R, Fahlström M. Prevalence of risk factors for cardiovascular disease stratified by body mass index categories in patients with wheelchair-dependent paraplegia after spinal cord injury. J Rehabil Med. 2012;44:440–3.
Rodriguez DJ, Benzel EC, Clevenger FW. The metabolic response to spinal cord injury. Spinal Cord. 1997;35:599–604.
Cox SA, Weiss SM, Posuniak EA, Worthington P, Prioleau M, Heffley G. Energy expenditure after spinal cord injury: an evaluation of stable rehabilitating patients. J Trauma. 1985;25:419–23.
Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77:371–8.
Webber CE. Reproducibility of DXA Measurements of Bone Mineral and Body Composition: Application to Routine Clinical Measurements. In: Handbook of Anthropometry. Springer, New York, NY, 2012, pp 151-65.
Illner K, Brinkmann G, Heller M, Bosy-Westphal A, Muller MJ. Metabolically active components of fat free mass and resting energy expenditure in nonobese adults. Am J Physiol Endocrinol Metab. 2000;278:E308–15.
Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition. 1993;61:223–44.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gomez JM, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23:1226–43.
Desport JC, Preux PM, Bouteloup-Demange C, Clavelou P, Beaufrere B, Bonnet C, et al. Validation of bioelectrical impedance analysis in patients with amyotrophic lateral sclerosis. Am J Clin Nutr. 2003;77:1179–85.
Buchholz AC, McGillivray CF, Pencharz PB. The use of bioelectric impedance analysis to measure fluid compartments in subjects with chronic paraplegia. Arch Phys Med Rehabil. 2003;84:854–61.
Yamada Y, Watanabe Y, Ikenaga M, Yokoyama K, Yoshida T, Morimoto T, et al. Comparison of single-or multifrequency bioelectrical impedance analysis and spectroscopy for assessment of appendicular skeletal muscle in the elderly. J Appl Physiol. 2013;115:812–8.
GE Laughton, AC Buchholz, KA Martin Ginis, RE Goy. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47:757–62.
SL Silveira, TA Ledoux, S Robinson-Whelen, R Stough, MA Nosek. Methods for classifying obesity in spinal cord injury: a review. Spinal Cord. 2017;55:812–17.
Davidsson L. Assessment of Body Composition and Total Energy Expenditure in Humans Using Stable Isotope Techniques: International Atomic Energy Agency, 2009.
Cornish B, Ward L. Data analysis in multiple-frequency bioelectrical impedance analysis. Physiol Meas. 1998;19:275.
De Lorenzo A, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol. 1997;82:1542–58.
Ward L, Isenring E, Dyer J, Kagawa M, Essex T. Resistivity coefficients for body composition analysis using bioimpedance spectroscopy: effects of body dominance and mixture theory algorithm. Physiol Meas. 2015;36:1529.
Matthie JR. Second generation mixture theory equation for estimating intracellular water using bioimpedance spectroscopy. J Appl Physiol. 2005;99:780–1.
Lin L, Torbeck LD. Coefficient of accuracy and concordance correlation coefficient: new statistics for methods comparison. Pda J Pharm Sci Technol. 1998;52:55–9.
Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–7.
Obesity: preventing and managing the global epidemic, World Health Organization, Geneva, Switzerland, 2000.
Laughton G, Buchholz A, Ginis KM, Goy R. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord 2009;47:757–62.
Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701.
Kocina P, Heyward V. Validation of a bioimpedance equation for estimating fat-free mass of spinal cord injured adults Med Sci Sports Exerc. 1997; 29: 55.
Vache C, Rousset P, Gachon P, Gachon AM, Morio B, Boulier A, et al. Bioelectrical impedance analysis measurements of total body water and extracellular water in healthy elderly subjects. Int J Obes Relat Metab Disord. 1998;22:537–43.
Lukaski HC, Johnson PE, Bolonchuk W, Lykken G. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985;41:810–7.
Segal KR, Burastero S, Chun A, Coronel P, Pierson RN Jr, Wang J. Estimation of extracellular and total body water by multiple-frequency bioelectrical-impedance measurement. Am J Clin Nutr. 1991;54:26–9.
Silveira S, Ledoux T, Robinson-Whelen S, Stough R, Nosek M. Methods for classifying obesity in spinal cord injury: a review. Spinal Cord. 2017;55:812–7.
Desport JC, Preux PM, Guinvarc’h S, Rousset P, Salle JY, Daviet JC, et al. Total body water and percentage fat mass measurements using bioelectrical impedance analysis and anthropometry in spinal cord-injured patients. Clin Nutr. 2000;19:185–90.
Mojtahedi M, Valentine R, Evans E. Body composition assessment in athletes with spinal cord injury: comparison of field methods with dual-energy X-ray absorptiometry. Spinal Cord. 2009;47:698–704.
Seoane F, Abtahi S, Abtahi F, Ellegård L, Johannsson G, Bosaeus I, et al. Mean expected error in prediction of total body water: a true accuracy comparison between bioimpedance spectroscopy and single frequency regression equations. BioMed Res Int. 2015;2015:656323.
Kim D, Park D, Lee B, Jeon J. A six-week motor-driven functional electronic stimulation rowing program improves muscle strength and body composition in people with spinal cord injury: a pilot study. Spinal Cord. 2014;52:621–4.
Panisset M, Galea M, El-Ansary D. Does early exercise attenuate muscle atrophy or bone loss after spinal cord injury? Spinal Cord. 2016;54:84–92.
Galea MP, Panisset MG, El-Ansary D, Dunlop SA, Marshall R, Clark JM, et al. SCIPA Switch-On: a randomized controlled trial investigating the efficacy and safety of functional electrical stimulation–assisted cycling and passive cycling initiated early after traumatic spinal cord injury. Neurorehabil Neural Repair. 2017;31:540–51.
Mulasi U, Kuchnia AJ, Cole AJ, Earthman CP. Bioimpedance at the bedside: current applications, limitations, and opportunities. Nutr Clin Pract. 2015;30:180–93.
Acknowledgements
We acknowledge the contributions of spinal consultant physicians Andrew Nunn and Douglas Brown of the Victorian Spinal Cord Service, as well as contributions of the participants and their families.
Author contributions
MGP participated in the design of the study, contributed to the design of the intervention protocol, contributed to grant procurement, performed data collection, performed data entry, contributed to statistical analyses, and drafted the manuscript. DK participated in the design of the study, contributed to grant procurement, performed data collection, performed data entry, and contributed to manuscript preparation. RJ participated in the design of the study, contributed to grant procurement, performed data collection, performed data entry, and contributed to manuscript preparation. RH participated in the design of the study, contributed to grant procurement, performed data collection, performed data entry, and contributed to manuscript preparation. RG performed data collection and contributed to manuscript preparation. WLC advised on the design of the data collection protocol, performed quality assurance on the data, contributed to statistical analyses and contributed to manuscript preparation. E-AD participated in the design of the study and contributed to the grant procurement and manuscript preparation. GMP developed the concept for and designed the study, contributed to grant procurement and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
WLC consults to ImpediMed Ltd. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Panisset, M.G., Desneves, K., Ward, L.C. et al. Bedside quantification of fat-free mass in acute spinal cord injury using bioelectrical impedance analysis: a psychometric study. Spinal Cord 56, 355–365 (2018). https://doi.org/10.1038/s41393-017-0035-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-017-0035-1
This article is cited by
-
A longitudinal analysis of resting energy expenditure and body composition in people with spinal cord injury undergoing surgical repair of pressure injuries: a pilot study
European Journal of Clinical Nutrition (2023)
-
Recent Updates in Nutrition After Spinal Cord Injury: 2015 Through 2021
Current Physical Medicine and Rehabilitation Reports (2022)
-
Comparison of segmental lean tissue mass in individuals with spinal cord injury measured by dual energy X-ray absorptiometry and predicted by bioimpedance spectroscopy
Spinal Cord (2021)
-
Comparison of estimated energy requirements using predictive equations with total energy expenditure measured by the doubly labelled water method in acute spinal cord injury
Spinal Cord (2019)
-
Spasticity and preservation of skeletal muscle mass in people with spinal cord injury
Spinal Cord (2019)