Abstract
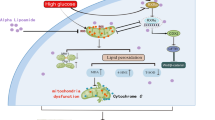
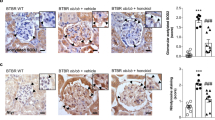
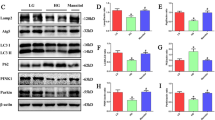
Diabetic kidney disease (DKD) is a common microvascular complication of diabetes mellitus, and oxidative stress and mitochondrial dysfunction play an important role in this process. It has been shown that aldose reductase (ALR2) catalyzes NADPH-dependent reduction of glucose to sorbitol, resulting in oxidative stress and mitochondrial dysfunction in diabetic patients. Astragalin (AG), a flavonoid extracted from Thesium chinense Turcz., shows an inhibitory activity on ALR2. In this study, we investigated the therapeutic effects of AG against renal injury in streptozocin (STZ)-induced diabetic mouse model. Diabetic mice were orally administered AG (5, 10 mg·kg−1·d−1) for 4 weeks. We showed that AG treatment greatly improved the proteinuria and ameliorated renal pathological damage without affecting the elevated blood glucose in diabetic mice. Furthermore, AG treatment significantly suppressed highly activated ALR2, and reduced oxidative stress in the kidney of diabetic mice and in high glucose and lipids-stimulated HK2 cells in vitro. We demonstrated that AG treatment modulated mitochondrial quality control and ameliorated apoptosis, boosting mitochondrial biogenesis, maintaining mitochondrial dynamic homeostasis, and improving energy metabolism disorder in vivo and in vitro. In high glucose and lipids-stimulated HK2 cells, we found that AG (20 μM) restored the phosphorylation level of AMPK, and upregulated the expression and transcriptional activity of PGC1α, whereas treatment with H2O2, blockade of AMPK with Compound C or knockdown of AMPKα with siRNA abolished the protective effect of AG on mitochondrial function, suggesting that antioxidant effects and activation of AMPK-dependent PGC1α pathway might be the molecular mechanisms underlying the protective effects of AG on mitochondrial quality control. We conclude that AG could be a promising drug candidate for the treatment of diabetic renal injury through activating AMPK.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Int J Endocrinol Metab. 2021;41:482–548.
Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol. 2018;14:291–312.
Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13:629–46.
Qin X, Jiang M, Zhao Y, Gong J, Su H, Yuan F, et al. Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br J Pharmacol. 2020;177:3646–61.
Qin X, Zhao Y, Gong J, Huang W, Su H, Yuan F, et al. Berberine protects glomerular podocytes via inhibiting Drp1-mediated mitochondrial fission and dysfunction. Theranostics. 2019;9:1698–713.
Yuan Y, Yuan L, Li L, Liu F, Liu J, Chen Y, et al. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1α activation. Stem Cells. 2021;39:913–28.
Terubayashi H, Sato S, Nishimura C, Kador PF, Kinoshita JH. Localization of aldose and aldehyde reductase in the kidney. Kidney Int. 1989;36:843–51.
Tang WH, Stitham J, Jin Y, Liu R, Lee SH, Du J, et al. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation. 2014;129:1598–609.
He J, Gao HX, Yang N, Zhu XD, Sun RB, Xie Y, et al. The aldose reductase inhibitor epalrestat exerts nephritic protection on diabetic nephropathy in db/db mice through metabolic modulation. Acta Pharmacol Sin. 2019;40:86–97.
Peng L, Gao X, Nie L, Xie J, Dai T, Shi C, et al. Astragalin attenuates dextran sulfate sodium (DSS)-induced acute experimental colitis by alleviating gut microbiota dysbiosis and inhibiting NF-κB activation in mice. Front Immunol. 2020;11:2058.
Kim YH, Kang MK, Lee EJ, Kim DY, Oh H, Kim SI, et al. Astragalin inhibits cigarette smoke-induced pulmonary thrombosis and alveolar inflammation and disrupts par activation and oxidative stress-responsive MAPK-signaling. Int J Mol Sci. 2021;22:3692.
Choung WJ, Hwang SH, Ko DS, Kim SB, Kim SH, Jeon SH, et al. Enzymatic synthesis of a novel kaempferol-3-o-β-d-glucopyranosyl-(1→4)-o-α-d-glucopyranoside using cyclodextrin glucanotransferase and its inhibitory effects on aldose reductase, inflammation, and oxidative stress. J Agric Food Chem. 2017;65:2760–7.
Liu CH, Chen HL, Zha XL, Zhu XX. Isolation and purification of aldose reductase and analysis of its properties. Acta Acad Med Shanghai. 1996;6:427–30.
Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 2018;108:656–62.
Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–35.
Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90.
Ricciardi CA, Gnudi L. Kidney disease in diabetes: from mechanisms to clinical presentation and treatment strategies. Metab Clin Exp. 2021;124:154890.
Schapira AH. Mitochondrial diseases. Lancet. 2012;379:1825–34.
Kou G, Li Z, Wu C, Liu Y, Hu Y, Guo L, et al. Citrus tangeretin improves skeletal muscle mitochondrial biogenesis via activating the AMPK-PGC1-α pathway in vitro and in vivo: a possible mechanism for its beneficial effect on physical performance. J Agric Food Chem. 2018;66:11917–25.
Kador PF, Wyman M, Oates PJ. Aldose reductase, ocular diabetic complications and the development of topical Kinostat®. Prog Retin Eye Res. 2016;54:1–29.
Tang WH, Martin KA, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87.
Kowalczyk P, Sulejczak D, Kleczkowska P, Bukowska OI, Kucia M, Popiel M, et al. Mitochondrial oxidative stress-a causative factor and therapeutic target in many diseases. Int J Mol Sci. 2021;22:13384.
Wang J, Zhu P, Li R, Ren J, Zhang Y, Zhou H. Bax inhibitor 1 preserves mitochondrial homeostasis in acute kidney injury through promoting mitochondrial retention of PHB2. Theranostics. 2020;10:384–97.
Tirichen H, Yaigoub H, Xu W, Wu C, Li R, Li Y. Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress. Front Physiol. 2021;12:627837.
Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100.
Wu SB, Wu YT, Wu TP, Wei YH. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim Biophys Acta. 2014;1840:1331–44.
Han X, Tai H, Wang X, Wang Z, Zhou J, Wei X, et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD+ elevation. Aging Cell. 2016;15:416–27.
Haemmerle G, Moustafa T, Woelkart G, Büttner S, Schmidt A, van de Weijer T, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med. 2011;17:1076–85.
Li SY, Susztak K. The role of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) in kidney disease. Semin Nephrol. 2018;38:121–6.
Dong YZ, Li L, Espe M, Lu KL, Rahimnejad S. Hydroxytyrosol attenuates hepatic fat accumulation via activating mitochondrial biogenesis and autophagy through the AMPK pathway. J Agric Food Chem. 2020;68:9377–86.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 82073859 and 81673658).
Author information
Authors and Affiliations
Contributions
MYS and HBW conceptualized the study. MYS and HJY contributed to the animal experiments. MYS and CZ contributed to the molecular biology experiments. MYS and ZJ contributed to the data collection and statistical analysis. YY contributed to the methodology. MYS and HBW drafted and finalized the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, My., Ye, Hj., Zheng, C. et al. Astragalin ameliorates renal injury in diabetic mice by modulating mitochondrial quality control via AMPK-dependent PGC1α pathway. Acta Pharmacol Sin 44, 1676–1686 (2023). https://doi.org/10.1038/s41401-023-01064-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01064-z
Keywords
This article is cited by
-
ALCAT1-mediated abnormal cardiolipin remodelling promotes mitochondrial injury in podocytes in diabetic kidney disease
Cell Communication and Signaling (2024)
-
Exploring a new mechanism between lactate and VSMC calcification: PARP1/POLG/UCP2 signaling pathway and imbalance of mitochondrial homeostasis
Cell Death & Disease (2023)