Abstract
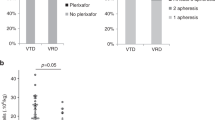
Bortezomib-based induction is often used in transplant-eligible patients with myeloma. The optimal peripheral blood stem cell (PBSC) mobilisation strategy in this context is unclear. We reviewed the efficacy of G-CSF alone (G-alone) vs. G-CSF and cyclophosphamide (G-cyclo: standard dose: 1.5–2 g/m2; high dose: 3–4 g/m2) PBSC mobilisation strategies in 288 patients who only received bortezomib, cyclophosphamide and dexamethasone (VCD) induction prior to autograft across six apheresis centres from November 2012 to June 2017. ‘Uncomplicated successful mobilisation’ was defined as achieving a PBSC yield of ≥4 × 106/kg within two aphereses, without plerixafor or mobilisation-associated toxicity (predominantly febrile neutropenia, FN). Success rates were 84% in G-cyclo standard dose (6% FN), 64% in G-cyclo high dose (18% FN) and 69% in G-alone (plerixafor successfully salvaged 8/9 patients). Median total stem cell yield was significantly higher with G-cyclo, but not different between the two cyclophosphamide doses. Age greater than the median of 61 years was associated with higher failure rates (22 vs. 11%, p = 0.01) and lower PBSC yield, especially in the G-alone group. Prior radiotherapy exposure did not impact on collection success. Our observations suggest that both G-cyclo standard dose and G-alone are reasonable mobilisation strategies. The former may be preferred if salvage plerixafor is unavailable.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ocio EM, Richardson PG, Rajkumar SV, Palumbo A, Mateos MV, Orlowski R, et al. New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia. 2014;28:525–42.
Quach H, Joshua D, Ho J, Szer J, Spencer A, Harrison SJ, et al. Treatment of patients with multiple myeloma who are eligible for stem cell transplantation: position statement of the Myeloma Foundation of Australia Medical and Scientific Advisory Group. Intern Med J. 2015;45:94–105.
Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114:1729–35.
Mohty M, Hubel K, Kroger N, Aljurf M, Apperley J, Basak GW, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49:865–72.
Gertz MA, Dingli D. How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood. 2014;124:882–90.
Oyekunle A, Shumilov E, Kostrewa P, Burchert A, Trumper L, Wuchter P, et al. Chemotherapy-based stem cell mobilisation does not result in significant paraprotein reduction in myeloma patients in the era of novel induction regimens. Biol Blood Marrow Transplant. 2017;24:276–81.
Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547–55.
Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337–41.
Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–53.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20:295–308.
Weaver CH, Birch R, Greco FA, Schwartzberg L, McAneny B, Moore M, et al. Mobilization and harvesting of peripheral blood stem cells: randomized evaluations of different doses of filgrastim. Br J Haematol. 1998;100:338–47.
Komeno Y, Kanda Y, Hamaki T, Mitani K, Iijima K, Ueyama J, et al. A randomized controlled trial to compare once- versus twice-daily filgrastim for mobilization of peripheral blood stem cells from healthy donors. Biol Blood Marrow Transplant. 2006;12:408–13.
Anderlini P, Donato M, Lauppe MJ, Huh YO, Martin TG, Chan KW, et al. A comparative study of once-daily versus twice-daily filgrastim administration for the mobilization and collection of CD34+ peripheral blood progenitor cells in normal donors. Br J Haematol. 2000;109:770–2.
Benyamini N, Avivi I, Dann EJ, Zuckerman T, Lavi N, Katz T. Comparison of engraftment following different stem cell mobilization modalities in patients with multiple myeloma treated with a uniform induction regimen containing bortezomib, cyclophosphamide and dexamethasone. Ann Hematol. 2017;96:461–7.
Civriz Bozdag S, Tekgunduz E, Altuntas F. The current status in hematopoietic stem cell mobilization. J Clin Apher. 2015;30:273–80.
Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18:1191–203.
Tuchman SA, Bacon WA, Huang LW, Long G, Rizzieri D, Horwitz M, et al. Cyclophosphamide-based hematopoietic stem cell mobilization before autologous stem cell transplantation in newly diagnosed multiple myeloma. J Clin Apher. 2015;30:176–82.
Olivieri A, Marchetti M, Lemoli R, Tarella C, Iacone A, Lanza F, et al. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo ItalianoTrapianto di Midollo Osseo. Bone Marrow Transplant. 2012;47:342–51.
Mohty M, Duarte RF, Croockewit S, Hubel K, Kvalheim G, Russell N. The role of plerixafor in optimizing peripheral blood stem cell mobilization for autologous stem cell transplantation. Leukemia. 2011;25:1–6.
Acknowledgements
We would like to acknowledge Ms Pina Polidano and Ms Narinder Kaur in helping with data acquisition.
Author contributions
CCC designed the study, acquired and analysed the data, and wrote the paper. HYL designed the study, acquired data and critically revised the paper. KLC, JO, SS, CW, MD, PC, JH, HK, CD, KB, SM, SG acquired data and critically revised the paper. AG designed the study, analysed the data and critically revised the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chua, C.C., Lim, H.Y., Chai, K.L. et al. Peripheral blood stem cell mobilisation with G-CSF alone versus G-CSF and cyclophosphamide after bortezomib, cyclophosphamide and dexamethasone induction in multiple myeloma. Bone Marrow Transplant 53, 1116–1123 (2018). https://doi.org/10.1038/s41409-018-0152-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0152-2
This article is cited by
-
Comparison of the efficiency, safety, and survival outcomes in two stem cell mobilization regimens with cyclophosphamide plus G-CSF or G-CSF alone in multiple myeloma: a meta-analysis
Annals of Hematology (2021)
-
The association of mobilising regimen on immune reconstitution and survival in myeloma patients treated with bortezomib, cyclophosphamide and dexamethasone induction followed by a melphalan autograft
Bone Marrow Transplantation (2021)
-
Cytarabine + G-CSF is more effective than cyclophosphamide + G-CSF as a stem cell mobilization regimen in multiple myeloma
Bone Marrow Transplantation (2019)